![]() Eduardo Mejia1
Eduardo Mejia1 ![]() ,
, ![]() Gustavo A. Ferraro1 and
Gustavo A. Ferraro1 and ![]() Igor C. A. Souto-Santos1
Igor C. A. Souto-Santos1
PDF: EN XML: EN | Supplementary: S1 S2 | Cite this article
Abstract
A new species of Rineloricaria from the São João and Macaé river basins in Rio de Janeiro State, southeastern Brazil is described. The new species is distinguished from most of its congeners by possessing of five series of lateral plates below the dorsal fin; mid-dorsal series consisting of four or five keeled plates extending posteriorly beyond the origin of the dorsal fin; pectoral girdle covered by plates; snout tip with naked area not reaching most anterior pore of infraorbital ramus of sensory canal; dorsal-fin spinelet present. The new species differs from R. zawadzkii, the most similar and geographically closest species, by having mid-ventral and lateral abdominal plates in contact (vs. separated by skin), the dorsal fin with a dark brown terminal band not reaching the edge, with inconspicuous dark dots along the lower edge (vs. band reaching the edge), and the caudal fin with a diffuse distal band with variegated white spots (vs. a well-defined distal band). The genetic distance based on cytochrome c oxidase I between the new species and the closest congeners supports its validity. The current distribution of the new species in the São João and Macaé basins is consistent with paleo-drainage connections influenced by sea-level fluctuations.
Keywords: DNA barcode, Freshwater fishes, Loricariinae, Sea-level variations, Suckermouth armored catfishes.
Introduction
The Neotropical genus Rineloricaria Bleeker, 1862 is widely distributed in Central and South America from southern Costa Rica to northern Argentina, inhabiting large rivers, streams, and ponds, from high mountain streams to large floodplain rivers (Reis, Cardoso, 2001; Fichberg, Chamon, 2008; Vera-Alcaraz et al.,2012). Rineloricaria is the most speciose genus of the subfamily Loricariinae with 74 valid species (Fricke et al., 2024). Rineloricaria is distinguished from other Loricariinae by the following characters: postorbital notch present; surface of lower lip with short round papillae; premaxilla with seven to 15 teeth on each ramus; dentary teeth strong, deeply bicuspidate; dorsal region with dark brown bars or blotches with the first one at the origin of dorsal fin; dorsal fin usually with the first element as a spinelet; abdomen with a polygonal preanal plate, usually bordered by three large trapezoidal plates (Fichberg, Chamon, 2008), and mid-dorsal series always present, varying from a short extension, reaching up to the origin of the dorsal-plate series to a longer extension, extending posteriorly below the dorsal fin (Mejia et al., 2023).
Currently, five valid species of Rineloricaria are documented in the Paraíba do Sul River and adjacent coastal basins: R. nudipectoris Mejia, Ferraro & Buckup, 2023, R. paraibensis Mejia & Buckup, 2024, and R. zawadzkii Costa-Silva, Silva & Oliveira, 2022, all described in the last three years. Additionally, R. nigricauda (Regan, 1904) and R. steindachneri (Regan, 1904) have been recently redescribed due to inadequate original descriptions (Mejia, Buckup, 2024). Previous studies have identified up to eight morphotypes of Rineloricaria for the Paraíba do Sul and coastal basins of southeastern Brazil (Bizerril, 1999; Bizerril, Primo, 2001). As part of a long-term study on the Rineloricaria diversity in the coastal drainages in southeastern Brazil, we recognized an undescribed species endemic to the São João and Macaé river basins. This new species is formally described herein based on molecular and morphological data.
Material and methods
Meristic and morphometric data. Studied specimens are available in the ichthyological collections of the Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro (MNRJ), and Museu de Ciências e Tecnologia da Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre (MCP). The samples include muscle tissue (tis) preserved in anhydrous ethanol. Counts and measurements were taken on the left side using digital calipers in millimeters (mm) and are given as percentages of standard length (SL), except for subunits of the head, which are expressed as percentages of head length (HL). Meristic and morphometric data followed Isbrücker, Nijssen (1978), and Vera-Alcaraz et al. (2012). Nomenclature and counts for the lateral-plate series followed Schaefer (1997) with modifications proposed by Vera-Alcaraz et al. (2012). Identification of mid-dorsal series followed Mejia et al. (2023: fig. 1). Terminology for anterior, median, and posterior plates of the abdominal complex followed Isbrücker, Nijssen (1978). Counts of dorsal-fin rays do not include the spinelet as the first unbranched ray. In the description, values within parentheses represent the total number of specimens corresponding to the associated counts, and asterisks indicate counts for holotype. In the lists of examined specimens, (type-material) museum acronyms and catalog numbers are followed by the number of specimens preserved in ethanol indicated as “alc”; the range of standard length; the number of specimens measured for morphometric analyses, with the range of standard length in parentheses and the number of specimens used for molecular data indicated as “tis”. Comparative material is listed by Mejia et al. (2023) and Mejia, Buckup (2024). Comparative data for Rineloricaria altipinnis (Breder, 1925), R. cachivera Urbano-Bonilla, Londoño-Burbano & Carvalho, 2023, R. caracasensis (Bleeker, 1862), R. daraha Rapp Py-Daniel & Fichberg, 2008, R. eigenmanni (Pellegrin, 1908), R. heteroptera Isbrücker & Nijssen, 1976, R. jubata (Boulenger, 1902), R. konopickyi (Steindachner, 1879), R. melini (Schindler, 1959), R. quilombola Chamon & Fichberg, 2022 and R. teffeana (Steindachner, 1879) were obtained from their original descriptions and/or high-resolution photographs of type-specimens available from Morris et al. (2006). Institutional abbreviations follow Sabaj (2020).
Molecular data. To support the morphological data, genomic DNA was extracted from muscle tissue preserved in anhydrous ethanol (Tab. S1) using the salting-out method (Miller et al., 1988). Partial sequences of the mitochondrial cytochrome oxidase subunit I (mt-co1) gene were amplified by Polymerase Chain Reaction (PCR) using primers FishF1 / FishR1 (Ward et al., 2005). The PCR protocol was as follows: denaturation at 94 °C/30 s, 35 cycles of 94°C/45 s, 50°C/30 s and 72°C/45 s, and a final step of 72°C/10 min. The amplified products were verified using 2% agarose gel electrophoresis. PCR products were purified using Exo-SAP (Handy et al., 2011). Each PCR product was sequenced bidirectionally on an ABI3730xl (Applied Biosystems) automated sequencer at the Fundação Oswaldo Cruz (FIOCRUZ). The forward and reverse sequencing chromatograms were manually edited and trimmed in BioEdit (Hall, 1999). Barcode Index Number (BIN; Ratnasingham, Hebert, 2007, 2013) was generated for the final sequences using the algorithm RESL (Refined Individual Linkage) available in Barcode of Life Data Systems (BOLD Systems, http://boldsystems.org/). Genetic divergence among populations and between the geographically closest species (Mejia, Buckup, 2024), was performed under the Kimura 2-parameter model (K2P, Kimura, 1980) with MEGA X (Kumar et al., 2018). To identify fish lineages as separate species we used the suggested DNA barcoding threshold of 2% (Pereira et al.,2013). New nucleotide sequences and associated data (chromatograms, geospatial coordinates, and primer details) are available under their respective Sample ID and Process ID codes in BOLD Systems and GenBank databases (see Tab. S1 for a full list of species and sequence accession codes).
Results
Rineloricaria buckupi, new species
urn:lsid:zoobank.org:act:8A342893-9540-4152-A28E-2947C7D63CDB
(Figs. 1–3; Tab. 1)
Holotype. MNRJ 51116, 111.2 mm SL, Brazil, Rio de Janeiro, Macaé, Macaé River, bridge on RJ-168 highway, 22°19’39”S 41°58’55”W, 11 Nov 2017, P. A. Buckup, V. de Brito, E. Malanski & R. M. Dias.
Paratypes. All from Brazil, Rio de Janeiro State: Macaé River basin: MCP 55326, 1 alc, 105.0 mm SL, 1 tis, Macaé, Ouro River, Forested section on Bicuda road, 22°17’15”S 42°00’36”W, 25 Nov 2011, P. A. Buckup, C. Quijada & J. C. Pascoli. MCP 55327, 1 alc, 104.8 mm SL, 1 tis, Rio Bonito, Rio Bacaxá River, near the bridge on BR-101 highway, 22°41’36”S 42°33’13”W, 14 Aug 2023, P. A. Buckup, I. C. A. Souto-Santos & J. E. Mejia de Loayza. MNRJ 51002, 4 alc, 46.3–107.5 mm SL (1, 107.5 mm SL), 2 tis, Conceição de Macabu, Aduelas Creek (Macaé drainage), under the bridge, near a sign written “Projeto Caminhos de Darwin”, 22°11’57”S 41°50’24”W, 17 Aug 2017, P. A. Buckup, D. F. Moraes Jr. & E. Malanski. MNRJ 55875, 1 alc, 155.9 mm SL, Casimiro de Abreu, bridge over the Macaé River near RJ-142 highway, 22°25’49”S 42°11’59”W, 20 Sep 2004, M. F. G. Brito. MNRJ 55876, 3 alc, 146.2–172.6 mm SL, Casimiro de Abreu, bridge over the Macaé River near RJ-142 highway, 22°25’49”S 42°11’59”W, 9 Jan 2005, M. F. G. Brito. MNRJ 55878, 4 alc, 132.6–141.1 mm SL, Casimiro de Abreu, bridge over the Macaé River near RJ-142 highway, 22°25’49”S 42°11’59”W, 9 Jan 2005, M. F. G. Brito. São João River basin: MNRJ 37445, 2 alc, 17.5–34.7 mm SL, Casimiro de Abreu, Aldeia Velha River, at bridge of BR-101 highway, 22°29’54”S 42°16’02”W, 21 Jul 2010, M. R. Britto, L. Villa-Verde & R. Bartolette. MNRJ 37475, 3 alc, 27.1–122.7 mm SL (1, 122.7 mm SL), 3 tis, Silva Jardim, Aldeia Velha River, under bridge on Aldeia Velha road, surroundings the RPPN Fazenda Bom Retiro, 22°28’03”S 42°17’51”W, 17 Jul 2010, M. R. Britto & F. Pupo. MNRJ 42777, 1 alc, 72.4 mm SL,1 tis, Silva Jardim, Imbau River, 2 km upstream from BR-101 highway, 22°37’10”S 42°28’02”W, 26 Feb 2014, P. A. Buckup, G. Beltrao & G. A. Ferraro. MNRJ 43012, 1 alc, 48.6 mm SL, 1 tis, Casimiro de Abreu, Lontras River, along BR-101 highway, 22°28’45”S 42°09’03”W, 27 Feb 2014, P. A. Buckup, G. Beltrão & G. A. Ferraro. MNRJ 52425, 1 alc, 46.3 mm SL, 1 tis, Silva Jardim, Maratuã River (tributary left bank of São João River) bridge on road between BR-101 highway and Bananeiras, 22°30’07”S 42°21’40”W, 2 Sep 2019, P. A. Buckup, I. C. A. Souto-Santos & G. A. Ferraro. MNRJ 54658, 6 alc, 55.3–101.8 mm SL (1, 101.8 mm SL), 2 tis, Rio Bonito, Bacaxá River, near bridge on BR-101 highway, 22°41’36”S 42°33’13”W, 14 Aug 2023, P. A. Buckup, I. C. A. Souto-Santos & J. E. Mejia de Loayza. MNRJ 54674, 10 alc, 37.2–99.3 mm SL (1, 99.3 mm SL), 3 tis, Casimiro de Abreu, Lontras River, on secondary road between Casimiro de Abreu and Professor Souza, 22°29’42”S 42°09’01”W, 14 Aug 2023, P. A. Buckup, I. C. A. Souto-Santos & J. E. Mejia de Loayza.
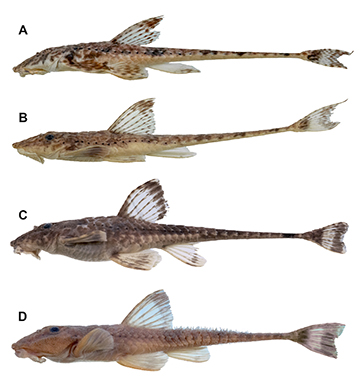
FIGURE 1| Rineloricaria buckupi, holotype, MNRJ 51116, 111.2 mm SL, Brazil, Macaé, Macaé River; lateral, dorsal, and ventral views.
Diagnosis. Rineloricaria buckupi can be distinguished from most congeners except for R. aequalicuspis Reis & Cardoso, 2001, R. altipinnis, R. anhaguapitan Ghazzi, 2008, R. anitae Ghazzi, 2008, R. baliola Rodriguez & Reis, 2008, R. cacerensis (Miranda Ribeiro, 1912), R. cachivera, R. caracasensis, R. capitonia Ghazzi, 2008, R. daraha, R. eigenmanni, R. fallax (Steindachner, 1915), R. formosa Isbrücker & Nijssen, 1979, R. hasemani Isbrücker & Nijssen, 1979, R. heteroptera, R. isaaci Rodriguez & Miquelarena, 2008, R. jaraguensis (Steindachner, 1909), R. jubata, R. konopickyi, R. latirostris (Boulenger, 1900), R. maacki Ingenito, Ghazzi, Duboc & Abilhoa, 2008, R. malabarbai Rodriguez & Reis, 2008, R. maquinensis Reis & Cardoso, 2001, R. melini, R. microlepidogaster (Regan, 1904), R. morrowi Fowler, 1940, R. nudipectoris, R. osvaldoi Fichberg & Chamon, 2008, R. pentamaculata Langeani & de Araujo, 1994, R. phoxocephala (Eigenmann & Eigenmann, 1889), R. platyura (Müller & Troschel, 1849), R. quilombola, R. reisi Ghazzi, 2008, R. rodriquezae Costa-Silva, Oliveira & Silva, 2021, R. rupestris (Schultz, 1944), R. steindachneri, R. stewarti (Eigenmann, 1909), R. teffeana, R. tropeira Ghazzi, 2008, R. zaina Ghazzi, 2008 and R. zawadzkii by having five lateral series of plates below the dorsal fin (vs. four lateral series of plates below the dorsal fin), and mid-dorsal series extending below and posterior to dorsal fin (vs. mid-dorsal series not extending beyond the origin of dorsal fin). Rineloricaria buckupi differs from R. aequalicuspis, R. anhaguapitan, R. baliola, R. capitonia, R. latirostris, R. maacki, R. malabarbai, R. maquinensis, R. microlepidogaster, R. nudipectoris, R. reisi, and R. tropeira by the extensive ventral covering of the pectoral girdle by plates (vs. absence of plates on most of the ventral surface of the pectoral girdle). It differs from R. cacerensis, R. fallax, R. formosa, R. hasemani, R. jubata, R. melini, R. morrowi, R. quilombola, R. teffeana, and R. zaina by the mid-dorsal series consisting of four or five keeled plates (vs. mid-dorsal series consisting of six to twelve keeled plates extending beyond the origin of the dorsal-fin base). Itdiffers from R. isaaci, R. jaraguensis, R. pentamaculata, R. rupestris, R. stewarti, and R. zawadzkii by the mid-ventral and lateral abdominal plates series in contact with each other (vs. series of mid-ventral and lateral abdominal plates separated by an area of skin). It differs from R. altipinnis, R. caracasensis, R. eigenmanni, R. heteroptera, R. konopickyi, R. osvaldoi, R. phoxocephala, and R. platyura by breeding males lacking hypertrophied odontodes on the dorsum of the head and predorsal region (vs. hypertrophied odontodes present on the dorsum of the head and predorsal region in breeding males). It differs from R. anitae and R. daraha by having three longitudinal series of plates in the median complex of abdominal plates (vs. several irregularly organized abdominal plates on the median complex in R. daraha; or five to six longitudinal series of abdominal plates on the median complex in R. anitae). It is distinguished from R. cachivera and R. rodriquezae, by its narrow head width 63.8–70.5% HL(vs. 73.5–93.5% HL in R. cachivera and 71.6–81.2% HL in R. rodriquezae). It is distinguished from R. steindachneri by wider cleithrum 15.9–18.1% SLand pelvic-fin margin reaching or surpassing anal-fin origin (vs. narrow cleithral width 13.4–15.8% SLand pelvic-fin margin never reaching the anal-fin origin).
Description. Morphometric data of holotype and paratypes in Tab. 1. Body strongly depressed at caudal peduncle, tapering posteriorly from pectoral fin. In lateral view, dorsal profile slightly convex from tip of the snout to origin of dorsal fin and relatively concave at dorsal base; straight from base of dorsal fin to caudal peduncle. Head length larger than cleithral width. In dorsal view, head elongated with triangular anterior profile. Snout tip with small naked area, not reaching the anteriormost pore of infraorbital ramus of sensory canal. Eye elliptical, with large, deep postorbital notch; iris operculum present. Lower lip covered by irregularly sized papillae randomly distributed around mouth. Short maxillary barbel; teeth bicuspid; dentary teeth larger than those of premaxilla; 6(5), 7*(10) teeth on premaxilla and dentary teeth 6(2), 7*(13) on dentary.
TABLE 1 | Morphometric data of holotype and paratypes of Rineloricaria buckupi. Range includes the holotype. N = number of specimens; SD = standard deviation.
| Holotype | N | Range | Mean | SD |
Standard length (mm) | 111.23 | 15 | 99.3–172.6 | – | – |
Percent of standard length | |||||
Head length | 23.6 | 15 | 22.8–24.9 | 23.8 | 0.7 |
Predorsal length | 33.4 | 15 | 32.6–35.1 | 33.7 | 0.7 |
Postdorsal length | 66.5 | 15 | 64.4–67.3 | 66.4 | 0.8 |
Prepectoral length | 18.9 | 15 | 18.0 –20.6 | 19.0 | 0.7 |
Postpectoral length | 82.7 | 15 | 81.2–84.7 | 83.0 | 1.1 |
Prepelvic length | 30.1 | 15 | 30.1–35.0 | 32.0 | 1.4 |
Postpelvic length | 70.1 | 15 | 66.3–71.4 | 69.4 | 1.2 |
Preanal length | 45.9 | 15 | 45.9–50.4 | 47.8 | 1.4 |
Postanal length | 54.0 | 15 | 50.1–54.4 | 52.9 | 1.3 |
Unbranched dorsal-fin ray | 21.1 | 15 | 18.2–21.1 | 20.0 | 0.8 |
Unbranched pectoral-fin ray | 15.8 | 15 | 13.2–17.4 | 15.7 | 1.2 |
Unbranched pelvic-fin ray | 14.7 | 15 | 13.5–16.1 | 14.7 | 0.8 |
Unbranched anal-fin ray | 17.8 | 15 | 15.1–18.3 | 16.9 | 0.9 |
Pectoral-pelvic fin distance | 12.4 | 15 | 12.4–15.8 | 13.9 | 0.9 |
Abdominal length | 16.9 | 15 | 16.0–18.1 | 17.2 | 0.7 |
Cleithral width | 16.0 | 15 | 15.9–18.1 | 16.7 | 0.6 |
Depth at dorsal-fin origin | 9.3 | 15 | 8.9–11.0 | 10.1 | 0.7 |
Width at anal-fin origin | 10.8 | 15 | 10.8–12.8 | 11.7 | 0.6 |
Caudal peduncle depth | 1.6 | 15 | 1.5–1.7 | 1.6 | 0.1 |
Caudal peduncle width | 3.2 | 15 | 2.8–3.4 | 3.2 | 0.1 |
Percent of head length | |||||
Snout length | 55.2 | 15 | 50.7–55.2 | 52.3 | 1.2 |
Eye diameter | 22.1 | 15 | 11.9–17.0 | 15.2 | 1.2 |
Maximum orbital diameter | 14.7 | 15 | 18.3–22.3 | 20.4 | 1.3 |
Interorbital width | 19.5 | 15 | 17.4–21.8 | 19.4 | 1.5 |
Internarial width | 7.4 | 15 | 6.1–8.0 | 7.4 | 0.5 |
Head depth | 35.6 | 15 | 33.8–40.1 | 36.9 | 1.8 |
Head width | 67.8 | 15 | 63.8–70.5 | 66.7 | 2.2 |
Free maxillary barbel | 9.0 | 14 | 5.3–11.8 | 7.7 | 1.9 |
Ventrorostral length | 6.1 | 15 | 4.3–9.2 | 6.3 | 1.1 |
Lower lip length | 17.2 | 15 | 11.2–18.0 | 14.3 | 2.1 |
Two predorsal plates between parieto-supraoccipital and nuchal plate with two low, inconspicuous ridges. Five (dorsal, mid-dorsal, median, mid-ventral, ventral) lateral plate series below dorsal fin. Dorsal series 20*(2), 21(5), 22(8) plates. Mid-dorsal series weakly keeled 4*(5), 5(10) plates. Median series 27*(3), 28(10), 29(2) plates. Lateral line complete. Mid-ventral plate series 15*(6), 16(6), 17(3) plates. Median and mid-ventral series with weak lateral ridge; these keels coalesce in last 11th(5), 12th*(8), 13th(2) plate of median series. Ventral series with 22*(5), 23(10). Lateral abdominal series with 7(11), 8*(4) plates between pectoral and pelvic-fin origin; number of plates on opposite sides of body sometimes different. Adjacent lateral and medial abdominal plates series in contact with each other. Abdomen entirely covered by plates, from opercular region to urogenital papilla. Posterior abdominal plate complex with large well-developed preanal plate anteriorly bordered by three polygonal plates, bordered by five plates. Middle abdominal plate complex, between lateral abdominal plates, with two or three longitudinal series posteriorly and four to six series anteriorly. Anterior complex of abdominal plates irregular and smaller anteriorly; anterior margin of complex with minute polygonal plates.
Dorsal-fin spinelet present. Unbranched dorsal-fin ray (dorsal spine) length shorter than head length (Tab. 1). Dorsal-fin origin slightly posterior to pelvic-fin origin; dorsal-fin rays i,7*(15); tip of dorsal fin when adpressed surpassing anal-fin origin, reaching eighth plate posterior to fin origin. Pectoral-fin rays i,6*(15); distal pectoral-fin margin slightly convex, when adpressed, surpassing pelvic-fin origin. Pelvic-fin rays i,5*(15); pelvic-fin margin reaching anal-fin origin; distal pelvic-fin margin convex; third branched ray slightly longer than unbranched pelvic-fin ray. Anal-fin rays i,5*(15); posterior anal-fin margin rounded; second branched ray longest. Caudal fin emarginated with i,10,i*(15) rays. Upper unbranched ray slightly longer than lower one, extended as short filament.
Coloration in alcohol. Background color of dorsal surface of head and body light brown. Ventral surface pale yellow. Sensory system pores on head and lateral medial plates notably darker. Five dark brown transverse bars on dorsal surface; first at dorsal-fin origin, second at tip of reclined dorsal fin, following bars located on caudal peduncle; third and fourth bars sometimes fused, forming four bars in some specimens (Fig. 2). Dorsal fin with dark brown terminal band not reaching border; terminal band with chromatophores concentrated on three unbranched rays on superior edge, becoming inconspicuous with dark dots aligned across inferior fin rays. Pectoral, pelvic, and anal fin covered with dark dots aligned across fin rays forming bands. Caudal fin with two vertical dark bands: broader solid one basally, one at distal margin, with variegated white spots. All fin membrane hyaline (Figs. 2–3).
FIGURE 2| Rineloricaria buckupi, paratypes. Note the remarkable dark brown transverse bars on dorsal surface. A. MNRJ 54658, 77.9 mm SL; five bars on dorsal surface. B. MNRJ 54658, 101.8 mm SL; notice third and fourth bars fused.
FIGURE 3| Comparison of chromatophores distribution in the bands on dorsal and caudal fin in Rineloricaria buckupi, MNRJ 51116, holotype, 111.2 mm SL (A), MNRJ 51002, paratype, 107.5 mm SL (B), and R. zawadzkii, MNRJ 37497, 122.1 mm SL (C), MNRJ 28475, 127.9 mm SL (D).
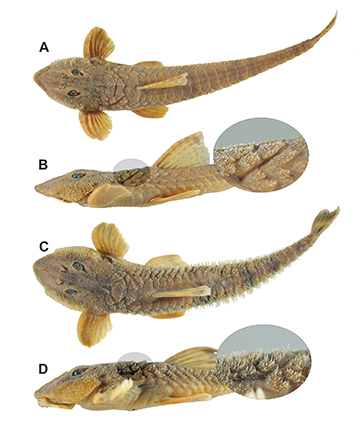
Sexual dimorphism. Mature males with abundant hypertrophied odontodes on the lateral margins of the head. Dorsal region of pectoral-fin rays covered almost entirely with thin, long odontodes with curved tips. Unbranched pectoral-fin ray thick, strongly curved, with short, densely arranged odontodes (Fig. 4).
FIGURE 4| Comparison of breeding males of Rineloricaria buckupi, MNRJ 55876, paratype, 147.7 mm SL (A, B), and R. zawadzkii, MNRJ 28475, 137.3 mm SL (C, D). Note the presence of hypertrophied odontodes on the lateral margins of the head and the thick unbranched pectoral-fin ray in R. buckupi, as well as hypertrophied odontodes on the dorsum of the head and predorsal region in R. zawadzkii.
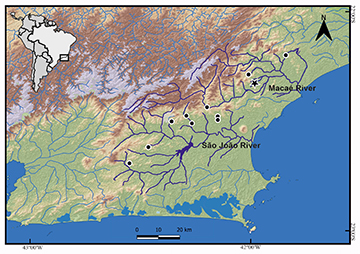
Geographical distribution. Rineloricaria buckupi is so far known from five tributaries of São João River (Aldeia Velha River, Bacaxá River, Capivari River, Lontra River, and Maratuã River) and two tributaries of the lower Macaé River (Ouro River and Aduelas Creek). São João and Macaé are coastal rivers flowing directly to the Atlantic Ocean, State of Rio de Janeiro, southeastern Brazil (Fig. 5). Specimens were collected from shallow streams (ca. 1–2 m deep) with variable current speeds and associated with sandy or muddy bottom with marginal submerged vegetation. Rineloricaria buckupi was recorded in syntopy with R. zawadzkii in the São João and Macaé basins. However, the new species appears to be restricted to the middle and lower Macaé River basin, with its farthest record at 22°25’49.20”S 42°11’59.58”W (64 km from its delta in the Atlantic Ocean), and does not extend to the upper region, where R. nudipectoris and R. zawadzkii are frequently recorded.
FIGURE 5| Partial map of southeastern Brazil (as indicated by the black square) showing the geographic distribution of Rineloricaria buckupi. Star represents the type-locality; black circles may represent more than one collection event in the same locality.
Etymology. The specific name buckupi (noun, masculine, singular genitive), is a patronym for Paulo A. Buckup, in recognition of his valuable teachings as an advisor to numerous students, including the authors of this paper. Paulo has done outstanding work and made numerous contributions to Neotropical ichthyology, including advances in the systematics of Rineloricaria over the past 25 years.
Conservation status. Rineloricaria buckupi is endemic to the São João and Macaé river basins in Rio de Janeiro State.Populations of this species are distributed across seven rivers tributaries of two unconnected basins (São João and Macaé, Fig. 5). Currently, no imminent threats to the species have been identified. Therefore, Rineloricaria buckupi is provisionally categorized as Least Concern (LC) according to the IUCN categories and criteria (IUCN Standards and Petitions Committee, 2024).
Molecular analysis. DNA barcodes based on the partial sequence of mt-co1 mitochondrial gene were generated for 16 specimens of Rineloricaria buckupi. Each river basin hosts a unique haplotype for the mt-co1 gene, with all specimens within the same basin sharing this specific haplotype; these haplotypes differ from each other by six mutations. The algorithm RESL (Refined Single Linkage) available in Barcode of Life Data Systems corroborated the separation of R. buckupi from the other geographically closest species included in this study. Rineloricaria buckupi received the Barcode Index Number BOLD:ADA2018.
The average genetic distance for the DNA barcode (mt-co1) between Rineloricaria buckupi and the closest described species R. zawadzkii was 4.0%. The new species also has a relatively high genetic distance from its other geographically closest congeners: R. nigricauda, R. nudipectoris, R. paraibensis, and R. steindachneri, with all values higher than 6.4% (Tab. 2). On the other hand, pairwise intraspecific distances in the new species were 0.0% within specimens from the same population and 0.9% between specimens from different populations (Tab. S2).
TABLE 2 | Pairwise genetic distance values for mitochondrial cytochrome oxidase c subunit 1 (mt-co1) gene between Rineloricaria buckupi and closest described species using a Kimura 2 Parameter model. Standard error estimates are shown above the diagonal and were obtained by a bootstrap procedure (100 pseudoreplicates).
|
| 1 | 2 | 3 | 4 | 5 | 6 |
1 | Rineloricaria buckupi |
| 0.009 | 0.009 | 0.009 | 0.011 | 0.01 |
2 | Rineloricaria zawadzkii | 0.04 |
| 0.009 | 0.009 | 0.012 | 0.01 |
3 | Rineloricaria nigricauda | 0.074 | 0.071 |
| 0.01 | 0.01 | 0.008 |
4 | Rineloricaria steindachneri | 0.065 | 0.057 | 0.082 |
| 0.012 | 0.01 |
5 | Rineloricaria nudipectoris | 0.072 | 0.077 | 0.068 | 0.091 |
| 0.012 |
6 | Rineloricaria paraibensis | 0.064 | 0.066 | 0.07 | 0.066 | 0.082 |
|
Discussion
The new species is assigned to the genus Rineloricaria based on the combination of morphological features proposed for the genus: postorbital notch present; surface of the lower lip with short round papillae; premaxilla with seven teeth on each ramus; dentary teeth strong, deeply bicuspidate; dorsal region with dark brown bars, dorsal fin with the first element as a spinelet; abdomen with a polygonal preanal plate, bordered by three large trapezoidal plates and mid-dorsal lateral plate series present (Fichberg, Chamon, 2008; Mejia et al., 2023). Rineloricaria buckupi is restricted to secondary tributaries of the São João and Macaé river basins, where it occurs in syntopy with R. zawadzkii.
The new species can be differentiated from Rineloricaria zawadzkii, the genetically closest species (Tab. 2), by the possession of mid-ventral and lateral abdominal plate series in contact with each other, and a dorsal fin with a dark brown terminal band not reaching the edge, becoming inconspicuous with dark dots aligned along the lower edge (Fig. 3; vs. series of mid-ventral and lateral abdominal plates separated by a wide post-pectoral naked area, and dorsal and caudal fins with a broad dark-brown terminal band reaching the edge). Additionally, the main diagnostic character of Rineloricaria zawadzkii is a strong secondary sexual dimorphism, with breeding males possessing hypertrophied odontodes covering the lateral and dorsal regions of the head and body (Figs. 4C, D; see Costa-Silva et al., 2022: fig. 1). In contrast, breeding males of Rineloricaria buckupi possess hypertrophied odontodes on the lateral margins of the head but lack them on the dorsum of the head and the predorsal region (Figs. 4A, B).
The average genetic distance between Rineloricaria buckupi and the closest described species (Tab. 2) is considerably higher than the thresholds suggested for delimiting fish species (2–3%; Ward, 2009; Pereira et al., 2013). In contrast, intraspecific genetic distances within populations are low, while genetic distances between Macaé and São João populations are relatively high (Tab. S2). The Macaé and São João basins are adjacent yet independent drainages emptying separately in the Atlantic Ocean. A reconstruction of the paleodrainages connections inferred for a sea level retreat of -125 m during the maximum glacial period of the Pleistocene (Thomaz, Knowles, 2018) suggests that these basins have been in the same paleodrainage (the Macaé-São João paleo-river), which would explain the current distribution of the new species and the observed genetic structure, which reflects a lack of gene flow due to the geographic isolation of these basins. The discovery of another undescribed species of whiptail catfish close to one of the largest metropolitan areas in Brazil further illustrates the incomplete knowledge about fish diversity. It highlights the need to document this unknown diversity before it faces potential extinction due to increasing human occupation.
Acknowledgments
The authors thank Roberto E. Reis and Carlos A. Lucena (MCP); Luiz R. Malabarba, André Netto-Ferreira, and Juliana Wingert (UFRGS) for providing access to specimens of Rineloricaria. We thank Marcelo Britto, Paulo Buckup, Durval Santos, and Anna Salles (MNRJ) for their curatorial and technical support. We thank Cristiano Moreira (MNRJ) for comments that improved the previous version of the manuscript, and Érica Caramaschi (UFRJ) for donating some male specimens. Lucas S. Medeiros (UFRN) provided the color style used in the altitudinal gradient of the map. This research was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (proc. to EM: CAPES/PROEX 88887.613913/2021–00; proc. to ICASS: CAPES/PRINT 88887.913513/2023–00), Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq grants (proc. to ICASS: 131148/2019–2, 140559/2021–3), and Fundação de Amparo à Pesquisa no Estado do Rio de Janeiro – FAPERJ grants (to Rosana Mazzoni as coordinator, E–26/210.053/2023).
References
Bizerril CRSF. A ictiofauna da bacia do Rio Paraíba do Sul. Biodiversidade e padrões biogeográficos. Braz Arch Biol Technol. 1999; 42(2):67–81. https://doi.org/10.1590/S1516-89131999000200014
Bizerril CRFS, Primo PBS. Peixes de águas interiores do Estado do Rio de Janeiro. Rio de Janeiro: FEMAR-SEMADS; 2001.
Costa-Silva GJ, Costa e Silva GS, Oliveira C. A new species of spiny Rineloricaria (Siluriformes: Loricariidae) from the Rio Paraíba do Sul basin and coastal rivers from Rio de Janeiro State. Zootaxa. 2022; 5175(2):285–92. https://doi.org/10.11646/zootaxa.5175.2.6
Fichberg I, Chamon CC. Rineloricaria osvaldoi (Siluriformes: Loricariidae): A new species of armored catfish from rio Vermelho, Araguaia basin, Brazil. Neotrop Ichthyol. 2008; 6(3):347–54. https://doi.org/10.1590/S1679-62252008000300008
Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2024. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acid S. 1999; 41:95–98.
Handy SM, Deeds JR, Ivanova NV, Hebert PDN, Hanner RH, Ormos A et al. A single-laboratory validated method for the generation of DNA barcodes for the identification of fish for regulatory compliance. J AOAC Int. 2011; 94(1):201–10. https://doi.org/10.1093/jaoac/94.1.201
International Union for Conservation of Nature (IUCN). Standards and Petitions Subcommittee. Guidelines for using the IUCN Red List categories and criteria, Version 15.1 [Internet], Gland; 2024. Available from: https://www.iucnredlist.org/resources/redlistguidelines
Isbrücker IJH, Nijssen H. Two new species and a new genus of neotropical mailed catfishes of the subfamily Loricariinae Swainson, 1838 (Pisces, Siluriformes, Loricariidae). Beaufortia. 1978; 27(339):177–206. Available from: https://repository.naturalis.nl/pub/504901
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 2018; 35(6):1547–49. https://doi.org/10.1093/molbev/msy096
Mejia E, Buckup PA. Species boundaries of the whiptail catfish Rineloricaria (Siluriformes: Loricariidae) from the Paraíba do Sul River drainage, southeastern Brazil, with species redescriptions and description of a new species. J Fish Biol. 2024; 105(1):288–313. https://doi.org/10.1111/jfb.15780
Mejia E, Ferraro GA, Buckup PA. A new species of Rineloricaria (Siluriformes: Loricariidae) from coastal drainages of Rio de Janeiro, southeastern Brazil. Neotrop Ichthyol. 2023; 21(1):e220083. https://doi.org/10.1590/1982-0224-2022-0083
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988; 16(3):1215. https://doi.org/10.1093/nar/16.3.1215
Morris PJ, Yager HM, Sabaj Pérez MH. ACSImagebase: a digital archive of catfish images compiled by participants in the All Catfish Species Inventory [Internet]. 2006. Available from: http://acsi.acnatsci.org/base
Pellegrin J. Description de deux poissons nouveaux de l’Amérique du Sud, de la famille des Loricariidés. Bull Soc Zool Fr.1908; 33:124–27. Available from: https://www.biodiversitylibrary.org/page/2161823#page/162/mode/1up
Pereira LHG, Hanner R, Foresti F, Oliveira C. Can DNA barcoding accurately discriminate megadiverse Neotropical freshwater fish fauna? BMC Genet. 2013; 14(20). https://doi.org/10.1186/1471-2156-14-20
Ratnasingham S, Hebert PDN. BOLD: The Barcode of Life Data System (www. barcodinglife.org). Mol Ecol Notes. 2007; 7(3):355–64. https://doi.org/10.1111/j.1471-8286.2007.01678.x
Ratnasingham S, Hebert PDN. A DNA based registry for all animal species: the Barcode Index Number (BIN) System. PLoS ONE. 2013; 8(8):e66213. https://doi.org/10.1371/journal.pone.0066213
Reis RE, Cardoso AR. Two new species of Rineloricaria from southern Santa Catarina and northeastern Rio Grande do Sul, Brazil (Teleostei: Loricariidae). Ichthyol Explor Freshw. 2001; 12(4):319–32.
Sabaj MH. Codes for natural history collections in ichthyology and herpetology. Copeia. 2020; 108(3):593–669. https://doi.org/10.1643/ASIHCODONS2020
Schaefer SA. The Neotropical cascudinhos: systematics and biogeography of the Otocinclus catfishes (Siluriformes: Loricariidae). Proc Acad Nat Sci Phila. 1997; 148:1–120. https://www.jstor.org/stable/4065046
Thomaz AT, Knowles LL. Flowing into the unknown: inferred paleodrainages for studying the ichthyofauna of Brazilian coastal rivers. Neotrop Ichthyol. 2018; 16(3):e180019. https://doi.org/10.1590/1982-0224-20180019
Vera-Alcaraz HS, Pavanelli CS, Zawadzki CH. Taxonomic revision of the Rineloricaria species (Siluriformes: Loricariidae) from the Paraguay River basin. Neotrop Ichthyol. 2012; 10(2):285–311. https://doi.org/10.1590/S1679-62252012000200006
Ward RD, Hanner R, Hebert PDN. The campaign to DNA barcode all fishes, FISHBOL. J Fish Biol. 2009; 74(2):329–56. https://doi.org/10.1111/j.1095-8649.2008.02080.x
Authors
![]() Eduardo Mejia1
Eduardo Mejia1 ![]() ,
, ![]() Gustavo A. Ferraro1 and
Gustavo A. Ferraro1 and ![]() Igor C. A. Souto-Santos1
Igor C. A. Souto-Santos1
[1] Programa de Pós-Graduação em Ciências Biológicas (Zoologia), Departamento de Vertebrados, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista, São Cristóvão, 20940-040 Rio de Janeiro, RJ, Brazil. (EM) edu.mejia@ufrj.br (corresponding author), (GAF) gustavoandresferraro@yahoo.com.br, (ICASS) icass.ufrj@gmail.com.Programa de Pós-Graduação em Ciências Biológicas (Zoologia), Departamento de Vertebrados, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista, São Cristóvão, 20940-040 Rio de Janeiro, RJ, Brazil. (EM) edu.mejia@ufrj.br (corresponding author), (GAF) gustavoandresferraro@yahoo.com.br, (ICASS) icass.ufrj@gmail.com.
Authors’ Contribution 

Eduardo Mejia: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing-original draft, Writing-review and editing.
Gustavo A. Ferraro: Data curation, Formal analysis, Investigation, Methodology, Writing-review and editing.
Igor C. A. Souto-Santos: Data curation, Formal analysis, Investigation, Methodology, Writing-review and editing.
Ethical Statement
This research was based on specimens previously preserved in official biological collections, and no living animals were handled in the laboratory.
Competing Interests
The author declares no competing interests.
How to cite this article
Mejia E, Ferraro GA, Souto-Santos ICA. A new species of whiptail catfish Rineloricaria (Siluriformes: Loricariidae) from Macaé and São João river basins, southeastern Brazil. Neotrop Ichthyol. 2025; 23(2):e240087. https://doi.org/10.1590/1982-0224-2024-0087
Copyright
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Distributed under
Creative Commons CC-BY 4.0

© 2025 The Authors.
Diversity and Distributions Published by SBI
![]() Accepted February 19, 2025
Accepted February 19, 2025
![]() Submitted August 26, 2024
Submitted August 26, 2024
![]() Epub May 26, 2026
Epub May 26, 2026