![]() Mauro Nirchio1
Mauro Nirchio1 ![]() ,
, ![]() Marcelo de Bello Cioffi2,
Marcelo de Bello Cioffi2, ![]() Francisco de Menezes Cavalcante Sassi2,
Francisco de Menezes Cavalcante Sassi2, ![]() Geize Aparecida Deon3,
Geize Aparecida Deon3, ![]() Claudio Oliveira3,
Claudio Oliveira3, ![]() Mariana Kuranaka3,
Mariana Kuranaka3, ![]() Jonathan Valdiviezo-Rivera4 and
Jonathan Valdiviezo-Rivera4 and ![]() Anna Rita Rossi5
Anna Rita Rossi5
PDF: EN XML: EN | Supplementary: S1 S2 S3 | Cite this article
Editor-in-chief: ![]() Carla Pavanelli
Carla Pavanelli
Abstract
Este estudo apresenta uma caracterização molecular e citogenética integrada de Chaetostoma bifurcum do Equador, pertencente à subfamília Hypostominae. A identificação morfológica foi confirmada por dados moleculares utilizando sequências dos genes da citocromo c oxidase subunidade I e citocromo b. A análise do cariótipo foi realizada com coloração convencional e hibridização in situ por fluorescência (FISH). O cariótipo é composto por 48 cromossomos metacêntricos/submetacêntricos e 6 subtelocêntricos/acrocêntricos, mantendo o número diploide (2n = 54) considerado ancestral na família Loricariidae, embora incomum em Hypostominae. Não foram observadas diferenças entre os cariótipos de fêmeas e machos, sugerindo ausência de cromossomos sexuais heteromórficos. Sequências teloméricas intersticiais (ITSs) tênues foram detectadas em um par submetacêntrico, cuja origem permanece incerta, podendo representar remanescentes de translocações decorrentes de mecanismos de reparo do DNA ou de DNA satélite. Os loci de rDNA 18S e 5S estão localizados em cromossomos distintos (pares 11 e 6, respectivamente), divergindo da sintenia plesiomórfica observada em Loricariidae. Esse padrão pode refletir tanto a história evolutiva quanto os efeitos de pressões ecológicas. Assim, análises comparativas com outras espécies do gênero Chaetostoma são essenciais para determinar se essas características cariotípicas refletem a posição filogenética ou processos adaptativos específicos, considerando que este estudo representa a primeira caracterização para o gênero.
Palavras-chave: Cascudos narigudos, Cromossomos, Filogenética, Fish, Genes ribossômicos.
Introduction
Loricariidae, commonly known as armored catfishes, represents a large family of freshwater fishes distributed across South and Central America. They are distinguished by their bony plates and suckermouths, which are specially adapted for feeding in fast-flowing, rheophilic environments (Covain, Fisch-Muller, 2007; Bressman et al., 2020). These fishes play a crucial ecological role, acting as specialized herbivores and consumers of organic matter. By controlling algae overgrowth, they help maintain the environmental balance within their habitats. Some Loricariidae species have been introduced into non-native freshwater ecosystems due to their popularity in the aquarium trade, where they can have negative impacts (Nico, Martin, 2001; Owsley et al., 2017; Orfinger, Goodding, 2018; Borzone Mas et al., 2019; Quintana et al., 2023). There are currently 1068 valid species within the Loricariidae, which is divided into six subfamilies: Lithogeninae, Delturinae, Rhinelepinae, Loricariinae, Hypoptopomatinae, and Hypostominae (Fricke et al., 2025). Hypostominae is the largest subfamily and comprises different tribes/clades whose relationships are still debated (Armbruster, 2004; Lujan et al., 2015a; Roxo et al., 2019). Hypostominae exhibited significant diversity in chromosome numbers and in the presence of differentiated sex chromosome systems (Fig. S1), although this variation is not uniformly distributed across tribes and genera (Sassi et al., 2024). Whitin Hypostominae, fishes of the genus Chaetostoma Tschudi 1846, generally attributed to the tribe Ancistrini and commonly known as rubbernose plecos, are described as the “Chatestoma clade” (Lujan et al., 2015a). This informal clade is sister of all other Hypostominae. Beyond species of Chaetostoma it contains a monophyletic sister taxon composed of species of Cordylancistrus Isbrücker, 1980, Dolichancistrus Isbrücker, 1980, and Leptoancistrus Meek & Hildebrand, 1916, distributed primarily in the northern Andes (Lujan et al., 2015b). Chaetostoma includes about 47 species, although its real diversity is likely underestimated (Lujan et al., 2015b) as demonstrated by recent identification and descriptions of new species (Urbano-Bonilla, Ballen, 2021; Meza-Vargas et al., 2022). These fishes are distributed along the Atlantic and Pacific slopes of the Andes Mountains, spanning from Panama to southern Peru, as well as in the Coastal Mountains of Venezuela, and various drainages within the Guiana and Brazilian shields (Lujan et al., 2015b).
As is the case with the ichthyofauna inhabiting streams and rivers of Andean Piedmont, Chaetostoma is threatened by the hydroelectric development (Finer, Jenkins, 2012). As a result, their preservation and conservation, together with their habitats, are of utmost importance to safeguard the ecological balance and maintain the rich biodiversity in these imperiled aquatic environments. According to the International Union for Conservation of Nature (IUCN), 15 Chaetostoma species are threatened of extinction. Of these, eight are designated as Endangered (EN) and seven as Vulnerable (VU) (IUCN, 2024).
Chaetostoma bifurcum Lujan, Meza-Vargas, Astudillo-Clavijo, Barriga Salazar & López-Fernández, 2015 (Fig. 1) is found in the Pacific Coast drainages of western Ecuador and northwestern Peru in piedmont elevations ranging from approximately 100 to 650 meters above sea level. Its distribution includes the Esmeraldas, Guayas, Santa Rosa, and Tumbes River drainages, from north to south (Lujan et al., 2015a). Unfortunately, the country’s aquatic ecosystems in this area are seriously threatened and circumstances are deteriorating (Aguirre et al., 2021). The species has not been evaluated by the IUCN and is unexplored in many biological and genetic aspects. In addition, there is no available data on any Chaetostoma species among the about 300 records (142 species) on chromosomal data for Hypostominae subfamily. Here we report the first cytogenetic data on C. bifurcum. Our objective is to ascertain whether chromosome number and karyotype structure exhibit the ancestral traits of the family/subfamily or, conversely, exhibit chromosomal rearrangements, as well as to investigate the presence of heteromorphic sex chromosomes in this species.
FIGURE 1| Chaetostoma bifurcum: dorsal (top), lateral (middle), and ventral (bottom) views. The bony plates covering the body and the distinct suckermouth, characteristic of the genus Chaetostoma, are visible, illustrating the adaptations of the species for adhering to rocky substrates in fast-flowing rivers. Scale bar = 10 mm.
Material and methods
Specimen collection. Twenty-four individuals of Chaetostoma bifurcum (9 males, 11 females, and 4 undetermined) were collected using a seine net in the Dos Bocas River (Cantón Pasaje), Ecuador. Fish were transported in sealed plastic bags, with the upper two-thirds of each bag filled with pure oxygen, ensuring optimal well-being to the Laboratories of Universidad Técnica de Machala until processing. The specimens were preliminarily identified based on external morphology (Lujan et al., 2015b) (Fig. 1), and this attribution was later validated on a subset of 11 specimens through molecular analysis. Sex determination was performed during dissection; sexually mature individuals could be reliably sexed based on gonadal morphology, whereas immature individuals were recorded as undetermined. Voucher specimens were fixed in a 10% formalin solution and deposited in the collection of the Instituto Nacional de Biodiversidad de Ecuador, under catalogue number MECN-DP 4962 (Tab. S2).
Specimen molecular identification. DNA extraction and polymerase chain reaction (PCR) amplification followed the procedures reported in Nirchio et al. (2023) using the Fish F1 and Fish R1 for cytochrome c oxidase I, COI (Ward et al., 2005) and cytbFa and cytbRa for cytochrome b, Cytb (Lujan et al., 2015a). Amplicons were purified and sequenced (Sanger method) using an external service (www.microsynth.ch). Sequences were aligned using Clustal X (Thompson et al., 2002), and the Basic Local Alignment Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to explore for Cytb and COI sequence similarity on the GenBank database and the Identification Request for COI sequences in BOLD System (https://v3.boldsystems.org).
Cytogenetic Analysis. Mitotic chromosome preparations were performed according to Nirchio et al. (2023). Chromosome were stained with Giemsa for morphology and the karyotype analysis. Metacentric (m) or submetacentric (sm) chromosomes were classified as bi-armed, and subtelocentric (st) or acrocentric (a) chromosomes as uniarmed (Levan et al., 1964). The C-banding procedure (Sumner, 1972) was applied for heterochromatin visualization; the silver staining method (Howell, Black, 1980) was used to identify nucleolus organizer regions (NORs).
Fluorescence in situ hybridization experiments. Genomic DNA from Chaetostoma bifurcum samples was isolated from the fin tissue preserved in 95% ethanol with the Wizard Genomic DNA Purification Kit (Promega), according to the manufacturer’s instructions. Subsequently, DNA was amplified via PCR using specific primers corresponding to the 5S rDNA (Pendas et al., 1995), 18S rDNA (Utsunomia et al., 2016), and telomeric probe (Ijdo et al., 1991). Using the nick-translation protocol (Jena Bioscience, Germany), the 5S rDNA and telomeric PCR-amplified sequences were labeled with Atto-550-dUTP and the 18S rDNA with Atto-425-dUTP. The FISH experiments followed the conditions described in Nirchio et al. (2023). To validate the results of in situ hybridization, at least 30 metaphase spreads were examined. Metaphases were captured in an Axioplan II fluorescence microscope (Carl Zeiss Jena GmbH, Germany) with the ISIS software (MetaSystems, Silver Spring, MD, USA).
Results
Molecular identification of specimens. COI sequences of 650 base pair (bp) corresponding to a single haplotype were obtained and deposited in GenBank (OR237843) (Nirchio et al., 2023). BLAST search (September 27, 2024) identified 98.45% similarity with Chaetostoma bifurcum, and similarity < 96% with several C. fisheri. In the BOLD System (accessed on September 27, 2024) there were no available sequences of C. bifurcum yet, and the highest similarity percentage was observed with C. fisheri. Cytb sequences (complete sequence, 1048 bp, a single haplotype. PV030915) confirmed species attribution and results of COI (99.7–99.5% similarity with C. bifurcum and < 96% with other congeneric species).
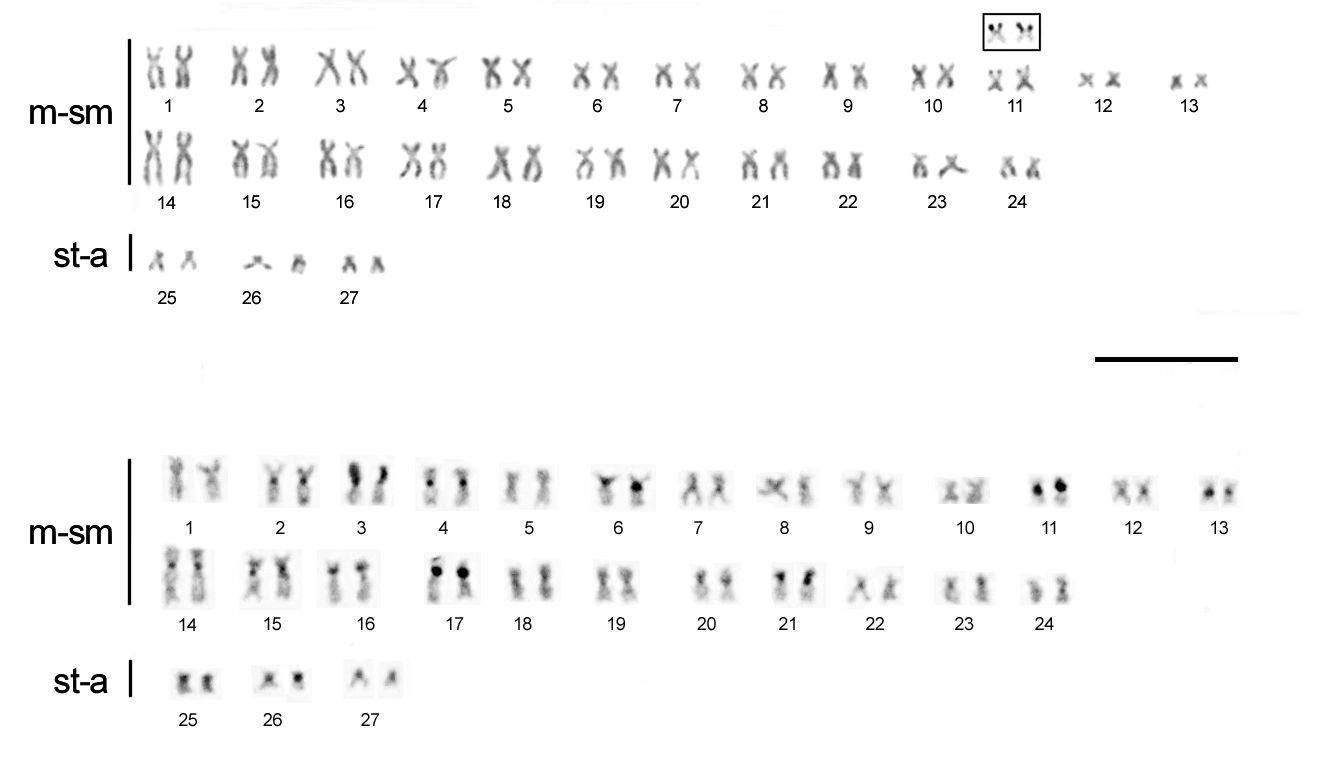
Cytogenetic analyses. Both males and females of Chaetostoma bifurcum possess a diploid complement of 54 chromosomes, with a karyotype composed of 48 m-sm + 6
st-a, and a fundamental number (FN) of 102 (Fig. 2). Silver nitrate impregnation revealed a unique pair of transcriptionally active nucleolus organizer regions (NORs), present at a distinct secondary constriction located on the short arm of the submetacentric pair 11 (Fig. 2A, boxed). C-banding revealed blocks of constitutive heterochromatin in all chromosomes, located in the centromeric and pericentromeric regions (Fig. 2B). A distinct heterochromatic block was consistently observed on the short arm of chromosome pair 3. No differences were found between males and females concerning their heterochromatin distribution.
FIGURE 2| Karyotype of Chaetostoma bifurcum stained with Giemsa (A), and after C-banding (B). The chromosome pair showing the nucleolus organizer region, after silver staining, is shown in the inset. m-sm, metacentric or submetacentric chromosomes; st-a subtelocentric or acrocentric chromosomes. Scale bar = 10 µm.
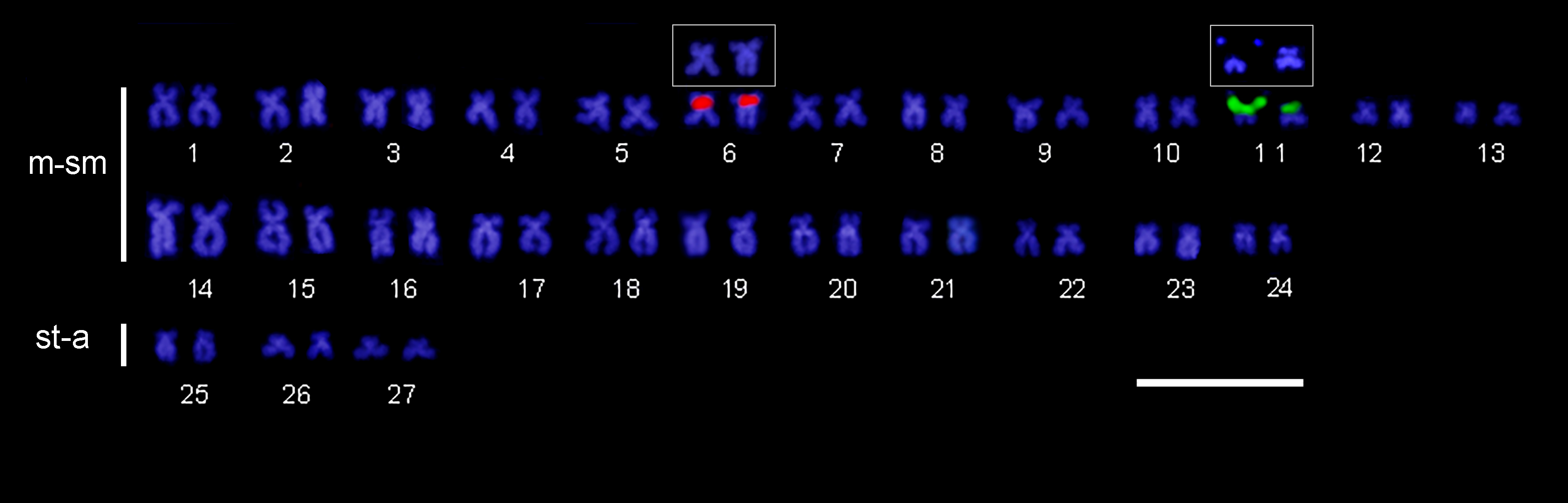
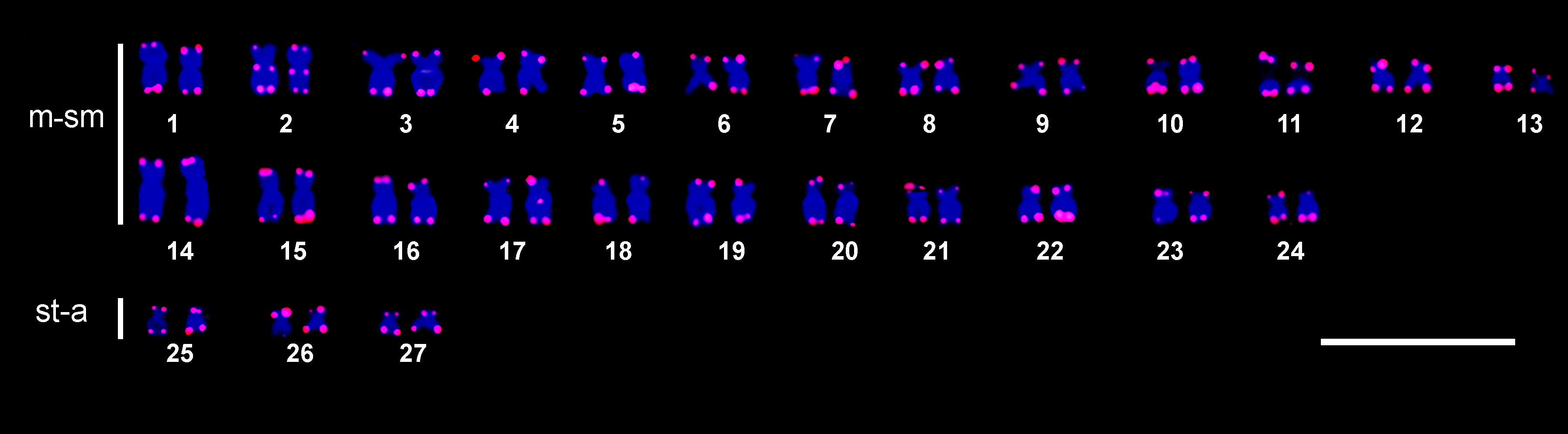
The fluorescence in situ hybridization (FISH) assay using the 18S rDNA probe confirmed the existence of a single large ribosomal cluster within the secondary constriction of the m-sm pair 11 (Fig. 3), which coincides with the NOR region. Moreover, the 5S rDNA probe revealed the presence of the minor ribosomal cistrons interstitially on the short arms of a middle-sized m-sm chromosome, likely pair 6. FISH with a telomeric probe shows positive signals in the terminal region of all chromosomes; in addition, a small interstitial telomeric sequence (ITS) was observed on a submetacentric chromosome pair (Fig. 4). No differences were recorded for any cytogenetic markers, between females and males.
FIGURE 3| Chaetostoma bifurcum karyotype after double fluorescence in situ hybridization with 18S rDNA (green) and 5S rDNA (red) probes. Box highlights the labeled pairs and their aspect in DAPI. Scale bar = 10 µm.
FIGURE 4| Chaetostoma bifurcum karyotype after fluorescence in situ hybridization using telomeric probes TTAGGGn. Faint ITSs are visible on chromosome pair number 2. Scale bar = 10 µm.
Discussion
Integrating molecular and cytogenetic approaches in teleost research has proven to be instrumental in uncovering the mechanisms underlying karyotype diversity and evolutionary pathways. These methodologies provide a framework to explore how genetic and evolutionary factors influence chromosomal rearrangements and diversification of species (Nirchio et al., 2014; Martinez et al., 2015; Supiwong et al., 2019; Moraes et al., 2021). In this study, we present the first cytogenetic data for Chaetostoma bifurcum, extending the number of Hypostominae species/genera analyzed. While our study focuses primarily on karyotypic patterns and their evolutionary implications, it sets the stage for future research aimed at exploring potential ecological correlates of karyotypic diversity within this group.
In Hypostominae, chromosome number ranges from 2n = 34 to 2n = 82 (Fig. S1). The tribes within the subfamily are not uniformly represented regarding species diversity, available research, or chromosome number (Fig. S3). For instance, the tribe Ancistrini is one of the two most studied, with 126 records spanning 16 genera, while Hypostomini includes 158 records from just two genera (Aphanotorulus Isbrücker & Nijssen, 1983and Hypostomus Lacepède, 1803). In contrast, Pterygoplichthini includes 13 records for the single genus, Pterygoplichthys Gill, 1858. The broad chromosome number range in Hypostominae is largely driven by two genera: Ancistrus Kner, 1854, with 2n ranging from 34 to 54 chromosomes (2n = 52 being the most common) and Hypostomus with 2n ranging from 66 to 82 (2n = 72 being the most common). Other genera and species, regardless of their tribe, typically share 2n = 52–54 chromosomes. This suggests that chromosome changes in the subfamily have not occurred uniformly during the evolutionary diversification of its genera (Cereali et al., 2008; Mariotto et al., 2011). In this context, although the phylogeny of Chaetostoma remains debated (Armbruster, 2004; Lujan et al., 2015a; Roxo et al., 2019) the genusis sister to all remaining Hypostominae, and the cytogenetic features of C. bifurcum are consistent with this interpretation. The species retains the ancestral diploid number proposed for Loricariidae (2n = 54) (Artoni, Bertollo, 2001), although this is not the most frequent chromosome number observed within Hypostominae (reviewed in Sassi et al., 2024). Further analyses of other Chaetostoma species within the genus are needed to determine whether this chromosome number is a general characteristic of the genus or a specific trait of C. bifurcum. Regarding sex chromosomes, none were detected in C. bifurcum, which is not unexpected given the apparent randomness of sex chromosome differentiation across Hypostominae even among closely related species (Glugoski et al., 2020).
Regarding the heterochromatin distribution, a conspicuous block was consistently observed on the short arm of chromosome pair 3. This block, clearly visible under conventional C-banding, may represent a species-specific cytogenetic feature. In other Ancistrini, extensive heterochromatic blocks have been linked to the accumulation of microsatellite repeats, such as (AC)₁₅ and (GT)₁₅, which are often interspersed within or adjacent to heterochromatic regions (Takagui et al., 2025). Moreover, certain microsatellites have been shown to interact with other repetitive DNA elements, such as rDNA sites, contributing to the establishment of evolutionary breakpoint regions that favor chromosomal rearrangements (Glugoski et al., 2018; Deon et al., 2022). Thus, we might hypothesize that the block observed in C. bifurcum reflects a preferential accumulation of microsatellites in that region. This hypothesis warrants further investigation through the physical mapping of specific microsatellite motifs on the karyotype of this species.
Different mechanisms may explain the presence of the ITSs, detected in C. bifurcum, which are absent in other Hypostominae: (a) ITSs are often interpreted as remnants of chromosomal rearrangements such as fusions or inversions (Ocalewicz, 2013). In C. bifurcum, where the diploid number (2n = 54) is the highest reported within Ancistrini, a fusion origin can be reasonably excluded, as fusions typically lead to a reduction in chromosome number. However, chromosomal inversions, which do not alter the chromosome count, remain a plausible explanation. (b) Another possible mechanism involves the generation of interstitial telomeric repeats through DNA repair processes. Comparative analyses of vertebrate genomes have shown that these sequences can arise independently of chromosomal rearrangements, as part of double-strand break repair pathways (Faravelli et al., 2002; Teixeira et al., 2016; Sola et al., 2021). This mechanism could account for the random presence of ITSs across closely related species, and thus be compatible with the absence of ITSs in other Ancistrini. (c) An additional source of ITSs are chromosomal rearrangements mediated by satellite DNA (Garrido-Ramos et al., 1998), although no information is currently available on the satellite DNA content of C. bifurcum, limiting the evaluation of this hypothesis. Although the ITS signal observed in C. bifurcum is relatively strong, even stronger than telomeric signals in the terminal regions of other chromosomes, its evolutionary and functional significance remains speculative. It is also possible that in related species, the absence of detectable ITSs results from technical limitations in identifying short interstitial sequences (Lund et al., 2009; Downs et al., 2012). Together, these findings emphasize the need for further comparative cytogenetic and genomic studies to better understand the origin and relevance of these chromosomal features.
Finally, we reflect on the rDNA localization pattern, which has long been recognized as a significant cytotaxonomic marker (Venere et al., 2008). Chaetostoma bifurcum possesses a single chromosome pair bearing 18S rDNA, a feature observed in the vast majority of Hypostominae, Loricariidae, and other teleost groups (Gornung, 2013), along with a single 5S rDNA site located on a different chromosome. This arrangement deviates from the plesiomorphic syntenic condition proposed for Loricariidae (Ziemniczak et al., 2012) but aligns with a more derived condition observed in Hypostominae, where 18S and 5S rDNA loci are commonly located on distinct chromosomes. On the other hand, rDNA positioning is also influenced by NOR-associated heterochromatin remodeling, which can drive interchromosomal exchange (Gornung, 2013). This indicates that rDNA mapping does not simply reflect lineage history but may also be shaped by additional factors. For example, differences in rDNA localization may arise from adaptation to environmental conditions (Silva et al., 2019), allowing species to optimize protein synthesis in response to ecological pressures (Cayuela et al., 2020), or by regulating rRNA and ribosome production (Kenmochi et al., 2001; Symonová, 2019; Hori et al., 2023). Furthermore, the adaptive potential of rDNA extends to others, extra ribosomal, functions: it may act as an early indicator of genomic instability or stress (Salim, Gerton, 2019). Therefore, the rDNA localization pattern observed in C. bifurcum likely results from both evolutionary divergence and local adaptive responses.
Since Arai’s (2011) comprehensive compilation, the number of karyotyped fish species has significantly increased, reflecting the growing application of cytogenetics in ichthyological research. This expansion has not only enhanced our understanding of chromosomal evolution across diverse lineages but has also proven instrumental in identifying cryptic species and uncovering hidden biodiversity (Cioffi et al., 2018). These advances underscore the continued significance of cytogenetic research in reconciling molecular and morphological methodologies, providing a more comprehensive understanding of the evolutionary history of teleost fishes.
Acknowledgments
We are grateful to Mr. Manuel Calderón, support staff from the Facultad de Ciencias Agropecuarias of Universidad Técnica de Machala, for his assistance in the field collection and maintenance of the specimens analyzed.
References
Aguirre WE, Alvarez-Mieles G, Anaguano-Yancha F, Burgos Morán R, Cucalón RV, Escobar-Camacho D et al. Conservation threats and future prospects for the freshwater fishes of Ecuador: a hotspot of Neotropical fish diversity. J Fish Biol. 2021; 99(4):1158–89. https://doi.org/10.1111/jfb.14844
Arai R. Fish karyotypes: a check list. Tokyo: Springer Science & Business Media; 2011.
Armbruster JW. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. Zool J Linn Soc. 2004; 141(1):1–80. https://doi.org/10.1111/j.1096-3642.2004.00109.x
Artoni RF, Bertollo LA. Trends in the karyotype evolution of Loricariidae fish (Siluriformes). Hereditas. 2001; 134(3):201–10. https://doi.org/10.1111/j.1601-5223.2001.00201.x
Borzone Mas D, Alvarenga PF, Scarabotti PA. Ecological and phylogenetic determinants of life-history patterns among ten loricariid species. J Fish Biol. 2019; 95(5):1298–310. https://doi.org/10.1111/jfb.14131
Bressman NR, Armbruster JW, Lujan NK, Udoh I, Ashley-Ross MA. Evolutionary optimization of an anatomical suction cup: Lip collagen content and its correlation with flow and substrate in Neotropical suckermouth catfishes (Loricarioidei). J Morphol. 2020; 281(6):676–87. https://doi.org/10.1002/jmor.21136
Cayuela H, Rougemont Q, Laporte M, Mérot C, Normandeau E, Dorant Y et al. Shared ancestral polymorphisms and chromosomal rearrangements as potential drivers of local adaptation in a marine fish. Mol Ecol. 2020; 29(13):2379–98. https://doi.org/10.1111/mec.15499
Cereali SS, Pomini E, Rosa R, Zawadzki CH, Froehlich O, Giuliano-Caetano L. Karyotype description of two species of Hypostomus (Siluriformes, Loricariidae) of the Planalto da Bodoquena, Brazil. Genet Mol Res. 2008; 7(3):583–91. https://doi.org/10.4238/vol7-3gmr404
Cioffi MB, Moreira-Filho O, Ráb P, Sember A, Molina WF, Bertollo LAC. Conventional cytogenetic approaches—useful and indispensable tools in discovering fish biodiversity. Curr Genet Med Rep. 2018; 6(4):176–86. https://doi.org/10.1007/s40142-018-0148-7
Covain R, Fisch-Muller S. The genera of the Neotropical armored catfish subfamily Loricariinae (Siluriformes: Loricariidae): a practical key and synopsis. Zootaxa. 2007; 1462(1):1–40. https://doi.org/10.11646/zootaxa.1462.1.1
Deon GA, Glugoski L, Hatanaka T, Sassi FMC, Nogaroto V, Bertollo LAC et al. Evolutionary breakpoint regions and chromosomal remodeling in Harttia (Siluriformes: Loricariidae) species diversification. Genet Mol Biol. 2022; 45(2):e20210170. https://doi.org/10.1590/1678-4685-GMB-2021-0170
Downs KP, Shen Y, Pasquali A, Beldorth I, Savage M, Gallier K et al. Characterization of telomeres and telomerase expression in Xiphophorus. Comp Biochem Physiol C Toxicol Pharmacol. 2012; 155(1):89–94. https://doi.org/10.1016/j.cbpc.2011.05.005
Faravelli M, Azzalin CM, Bertoni L, Chernova O, Attolini C, Mondello C et al. Molecular organization of internal telomeric sequences in Chinese hamster chromosomes. Gene. 2002; 283(1–2):11–16. https://doi.org/10.1016/s0378-1119(01)00877-0
Finer M, Jenkins CN. Proliferation of hydroelectric dams in the Andean Amazon and implications for Andes-Amazon connectivity. PLoS ONE. 2012; 7(4):e35126. https://doi.org/10.1371/journal.pone.0035126
Fricke R, Eschmeyer WN, Van der Laan R. Eschemeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2025. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Garrido-Ramos MA, Herrán R, CR Rejón, MR Rejón. A satellite DNA of the Sparidae family (Pisces, Perciformes) associated with telomeric sequences. Cytogenet Cell Genet. 1998; 83(1–2):3–09. https://doi.org/10.1159/000015151
Glugoski L, Deon G, Schott S, Vicari MR, Nogaroto V, Moreira-Filho O. Comparative cytogenetic analyses in Ancistrus species (Siluriformes: Loricariidae). Neotrop Ichthyol. 2020; 18(2):e200013. https://doi.org/10.1590/1982-0224-2020-0013
Gornung E. Twenty years of physical mapping of major ribosomal RNA genes across the teleosts: a review of research. Cytogenet Genome Res. 2013; 141(2–3):90–102. https://doi.org/10.1159/000354832
Hori Y, Engel C, Kobayashi T. Regulation of ribosomal RNA gene copy number, transcription and nucleolus organization in eukaryotes. Nat Rev Mol Cell Biol. 2023; 24(6):414–29. https://doi.org/10.1038/s41580-022-00573-9
Howell WM, Black DA. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia. 1980; 36(8):1014–15. https://doi.org/10.1007/BF01953855
Ijdo JW, Wells RA, Baldini A, Reeders ST. Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res. 1991; 19(17):4780. https://doi.org/10.1093/nar/19.17.4780
International Union for Conservation of Nature (IUCN). The IUCN Red List of Threatened Species. Version 2024 [Internet]. Cambridge; 2024. Available from: https://www.iucnredlist.org
Kenmochi N, Suzuki T, Uechi T, Magoori M, Kuniba M, Higa S et al. The human mitochondrial ribosomal protein genes: mapping of 54 genes to the chromosomes and implications for human disorders. Genomics. 2001; 77(1–2):65–70. https://doi.org/10.1006/geno.2001.6622
Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964; 52(2):201–20. https://doi.org/10.1111/j.1601-5223.1964.tb01953.x
Lujan NK, Armbruster JW, Lovejoy NR, López-Fernández H. Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Mol Phylogenet Evol. 2015a; 82:269–88. https://doi.org/10.1016/j.ympev.2014.08.020
Lujan NK, Meza-Vargas V, Astudillo-Clavijo V, Barriga-Salazar R, López-Fernández H. A multilocus molecular phylogeny for Chaetostoma clade genera and species with a review of Chaetostoma (Siluriformes: Loricariidae) from the central Andes. Copeia. 2015b; 103(3):664–701. https://doi.org/10.1643/ci-14-194
Lund TC, Glass TJ, Tolar J, Blazar BR. Expression of telomerase and telomere length are unaffected by either age or limb regeneration in Danio rerio. PLoS ONE. 2009; 4(11):e7688. https://doi.org/10.1371/journal.pone.0007688
Mariotto S, Centofante L, Vicari MR, Artoni RF, Moreira-Filho O. Chromosomal diversification in ribosomal DNA sites in Ancistrus Kner, 1854 (Loricariidae, Ancistrini) from three hydrographic basins of Mato Grosso, Brazil. Comp Cytogenet. 2011; 5(4):289–300. https://doi.org/10.3897/CompCytogen.v5i4.1757
Martinez PA, Zurano JP, Amado TF, Penone C, Betancur-R R, Bidau CJ et al. Chromosomal diversity in tropical reef fishes is related to body size and depth range. Mol Phylogenet Evol. 2015; 93:1–04. https://doi.org/10.1016/j.ympev.2015.07.002
Meza-Vargas V, Calegari BB, Lujan NK, Ballen GA, Oyakawa OT, Sousa LM et al. A new species of Chaetostoma (Siluriformes: Loricariidae) expands the distribution of rubbernose plecos eastward into the lower Amazon basin of Brazil. Ichthyol Herpetol. 2022; 110(2):364–74. https://doi.org/10.1643/i2021068
Moraes RLR, Sassi FMC, Bertollo LAC, Marinho MMF, Viana PF, Feldberg E et al. Tracking the evolutionary trends among small-size fishes of the genus Pyrrhulina (Characiforme, Lebiasinidae): new insights from a molecular cytogenetic perspective. Front Genet. 2021; 12:769984. https://doi.org/10.3389/fgene.2021.769984
Nico LG, Martin RT. The South American suckermouth armored catfish, Pterygoplichthys anisitsi (Pisces: Loricaridae), in Texas, with comments on foreign fish introductions in the American southwest. Southwest Nat. 2001; 46(1):98–104. https://doi.org/10.2307/3672381
Nirchio M, Cioffi MB, Sassi FMC, Deon GA, Oliveira C, Kuranaka M et al. Integrating genomic and chromosomal data: a cytogenetic study of Transancistrus santarosensis (Loricariidae: Hypostominae) with characterization of a ZZ/ZW sex chromosome system. Genes. 2023a; 14(9):1662. https://doi.org/10.3390/genes14091662
Nirchio M, Rossi AR, Foresti F, Oliveira C. Chromosome evolution in fishes: a new challenging proposal from Neotropical species. Neotrop Ichthyol. 2014; 12(4):761–70. https://doi.org/10.1590/1982-0224-20130008
Ocalewicz K. Telomeres in fishes. Cytogenet Genome Res. 2013; 141(2–3):114–25. https://doi.org/10.1159/000354278
Orfinger AB, Goodding DD. The global invasion of the suckermouth armored catfish genus Pterygoplichthys (Siluriformes: Loricariidae): annotated list of species, distributional summary, and assessment of impacts. Zool Stud. 2018; 57:e7. https://doi.org/10.6620/ZS.2018.57-07
Owsley CM, Neleigh CE, Lee Vaughan M, Castiglione JD, Distel CA. Preliminary Report: Exotic armored catfish may reduce survival and growth of native amphibians. Bios. 2017; 88(2):86–91. https://doi.org/10.1893/BIOS-D-16-00005.1
Pendas AM, Moran P, Martinez JL, Garcia-Vazquez E. Applications of 5S rDNA in Atlantic salmon, brown trout, and in Atlantic salmon brown trout hybrid identification. Mol Ecol. 1995; 4(2):275–76. https://doi.org/10.1111/j.1365-294X.1995.tb00220.x
Quintana Y, Keppeler FW, Winemiller KO. Does invasion by armored catfish shift trophic ecology of native fishes? Evidence from stable isotope analysis. Ecology. 2023; 104(5):e4024. https://doi.org/10.1002/ecy.4024
Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC et al. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phylogenet Evol. 2019; 135:148–65. https://doi.org/10.1016/j.ympev.2019.02.017
Salim D, Gerton JL. Ribosomal DNA instability and genome adaptability. Chromosome Res. 2019; 27(1–2):73–87. https://doi.org/10.1007/s10577-018-9599-7
Sassi FMC, Cioffi MB, Moreira-Filho O. A state-of-art review of Loricariidae (Ostariophysi: Siluriformes) cytogenetics. Neotrop Ichthyol. 2024; 22(4):e240050. https://doi.org/10.1590/1982-0224-2024-0050
Silva FA, Feldberg E, Moura Carvalho ND, Hernández Rangel SM, Schneider CH, Carvalho-Zilse GA et al. Effects of environmental pollution on the rDNAomics of Amazonian fish. Environ Pollut. 2019; 252:180–87. https://doi.org/10.1016/j.envpol.2019.05.112
Sola L, Nergadze SG, Cappelletti E, Piras FM, Giulotto E, Santagostino M. Telomeric-like repeats flanked by sequences retrotranscribed from the telomerase RNA inserted at DNA double-strand break sites during vertebrate genome evolution. Int J Mol Sci. 2021; 22(20):11048. https://doi.org/10.3390/ijms222011048
Sumner AT. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 1972; 75:304–06. https://doi.org/10.1016/0014-4827(72)90558-7
Supiwong W, Pinthong K, Seetapan K, Saenjundaeng P, Bertollo LAC, Oliveira EA et al. Karyotype diversity and evolutionary trends in the Asian swamp eel Monopterus albus (Synbranchiformes, Synbranchidae): a case of chromosomal speciation? BMC Evol Biol. 2019; 19(1):73. https://doi.org/10.1186/s12862-019-1393-4
Symonová R. Integrative rDNAomics-Importance of the oldest repetitive fraction of the eukaryote genome. Genes. 2019; 10(5):345. https://doi.org/10.3390/genes10050345
Takagui FH, Santana LP, Rubert M, Viana P, Affonso PRAM, Giuliano-Caetano L. The role of dispersal of repetitive DNAs in the diversification of bristlenose plecos (Loricariidae, Hypostominae, Ancistrus) from South Atlantic Coastal drainages. An Acad Bras Cienc. 2025; 97(1):e20240901. https://doi.org/10.1590/0001-3765202520240901
Teixeira LSR, Seger KR, Targueta CP, Orrico VGD, Lourenço LB. Comparative cytogenetics of tree frogs of the Dendropsophus marmoratus (Laurenti, 1768) group: conserved karyotypes and interstitial telomeric sequences. Comp Cytogenet. 2016; 10(4):753–67. https://doi.org/10.3897/CompCytogen.v10i4.9972
Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics. 2002; 2(1):2.3. https://doi.org/10.1002/0471250953.bi0203s00
Urbano-Bonilla A, Ballen GA. A new species of Chaetostoma (Siluriformes: Loricariidae) from the Orinoco basin with comments on Amazonian species of the genus in Colombia. J Fish Biol. 2021; 98(4):1091–104. https://doi.org/10.1111/jfb.14640
Utsunomia R, Silva DMZA, Ruiz-Ruano FJ, Araya-Jaime C, Pansonato-Alves JC, Scacchetti PC et al. Uncovering the ancestry of B chromosomes in Moenkhausia sanctaefilomenae (Teleostei, Characidae). PLoS ONE. 2016; 11(3):e0150573. https://doi.org/10.1371/journal.pone.0150573
Venere PC, Souza IL, Silva LKS, Anjos MB, Oliveira RR, Galetti Jr. PM. Recent chromosome diversification in the evolutionary radiation of the freshwater fish family Curimatidae (Characiformes). J Fish Biol. 2008; 72(8):1976–89. https://doi.org/10.1111/j.1095-8649.2008.01814.x
Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN. DNA barcoding Australia’s fish species. Philos Trans R Soc Lond B Biol Sci. 2005; 360(1462):1847–57. https://doi.org/10.1098/rstb.2005.1716
Ziemniczak K, Barros AV, Rosa KO, Nogaroto V, Almeida MC, Cestari MM et al. Comparative cytogenetics of Loricariidae (Actinopterygii: Siluriformes): Emphasis in Neoplecostominae and Hypoptopomatinae. Ital J Zool. 2012; 79(4):492–501. https://doi.org/10.1080/11250003.2012.676677
Authors
![]() Mauro Nirchio1
Mauro Nirchio1 ![]() ,
, ![]() Marcelo de Bello Cioffi2,
Marcelo de Bello Cioffi2, ![]() Francisco de Menezes Cavalcante Sassi2,
Francisco de Menezes Cavalcante Sassi2, ![]() Geize Aparecida Deon3,
Geize Aparecida Deon3, ![]() Claudio Oliveira3,
Claudio Oliveira3, ![]() Mariana Kuranaka3,
Mariana Kuranaka3, ![]() Jonathan Valdiviezo-Rivera4 and
Jonathan Valdiviezo-Rivera4 and ![]() Anna Rita Rossi5
Anna Rita Rossi5
[1] Departamento de Acuicultura, Universidad Técnica de Machala, Av. Panamericana km 5.5, Vía Pasaje, Machala 070150, El Oro, Ecuador. (MN) mauro.nirchio@gmail.com (corresponding author).
[2] Departamento de Genética e Evolução, Universidade Federal de São Carlos, 13565-090 São Carlos, SP, Brazil. (MBC) mbcioffi@ufscar.br, (FMCS) francisco.sassi@hotmail.com, (GAD) geizeadeon@gmail.com.
[3] Departamento de Biologia Estrutural e Funcional, Instituto de Biociências, Universidade Estadual Paulista-UNESP, 18618-689 Botucatu, SP, Brazil. (CO) claudio.oliveira@unesp.br, (MK) mariana.kuranaka@unesp.br.
[4] Instituto Nacional de Biodiversidad, Rumipamba, 341, Av. Shyris, Parque La Carolina, Quito, Ecuador. (JVR) bioictiojona@yahoo.com.
[5] Dipartimento di Biologia e Biotecnologie “C. Darwin”, Sapienza – Università di Roma, Via Alfonso Borelli 50, 00161 Rome, Italy. (ARR) annarita.rossi@uniroma1.it.
Authors’ Contribution 

Mauro Nirchio: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing-original draft, Writing-review and editing.
Marcelo de Bello Cioffi: Conceptualization, Formal analysis, Funding acquisition, Resources, Writing-original draft, Writing-review and editing.
Francisco de Menezes Cavalcante Sassi: Formal analysis, Methodology, Writing-original draft, Writing-review and editing.
Geize Aparecida Deon: Formal analysis, Methodology, Writing-review and editing.
Claudio Oliveira: Conceptualization, Formal analysis, Funding acquisition, Investigation, Validation, Writing-original draft, Writing-review and editing.
Mariana Kuranaka: Methodology, Writing-original draft, Writing-review and editing.
Jonathan Valdiviezo-Rivera: Methodology, Writing-original draft, Writing-review and editing.
Anna Rita Rossi: Conceptualization, Formal analysis, Funding acquisition, Methodology, Validation, Writing-original draft, Writing-review and editing.
Ethical Statement
All procedures executed within this study were conducted with the proper authorization granted by the Institutional Research Project (2024/UTMACH-PR-GEN-278). Furthermore, all activities involving experimental animals were conducted in strict accordance with the ethical standards outlined by the Universidad Técnica de Machala’s Ethics Committee on Animal Experimentation, as indicated by the official process number UTMACH-CEEA-014/2024.
Competing Interests
The author declares no competing interests.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Funding
This research was supported by Universidad Técnica de Machala (Grant 2024/UTMACH-PR-GEN–278) to MN; FAPESP (Grants 2023/00955–2 and 2020/13433–6) to MdBC and CO, respectively; CNPq (Grants 302928/2021–9 and 405706/2022–7) to MdBC, and (Grants 306054/2006–0 and 441128/2020–3) to CO; Prope-UNESP to CO; and Sapienza Università di Roma (Grant RM1221816815568D) to ARR.
How to cite this article
Nirchio M, Cioffi MB, Sassi FMC, Deon GA, Oliveira C, Kuranaka M, Valdiviezo-Rivera J, Rossi AR. Cytogenetic and molecular identification of Chaetostoma bifurcum (Siluriformes: Loricariidae) from the Pacific Coast of Ecuador. Neotrop Ichthyol. 2025; 23(3):e250021. https://doi.org/10.1590/1982-0224-2025-0021
Copyright
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Distributed under
Creative Commons CC-BY 4.0

© 2025 The Authors.
Diversity and Distributions Published by SBI
![]() Accepted June 10, 2025
Accepted June 10, 2025
![]() Submitted February 6, 2025
Submitted February 6, 2025
![]() Epub September 29, 2025
Epub September 29, 2025