Carmen G. Montaña1 ![]()
![]() , Elford Liverpool2
, Elford Liverpool2 ![]() , Donald C. Taphorn3
, Donald C. Taphorn3 ![]() and Christopher M. Schalk4
and Christopher M. Schalk4![]()
PDF: EN XML: EN | Cite this article
Abstract
In South America, mercury contamination due to gold mining operations is a threat to both biodiversity and human health. We examined mercury (Hg) concentrations in fishes that constitute important subsistence fisheries from mined and non-mined tributaries in the middle Mazaruni River, Guyana. Mercury concentrations and trophic food web structure (based on carbon and nitrogen stable isotopes) were characterized for primary basal sources and 39 fish species representing seven trophic guilds. Fishes collected at mined sites had higher mercury concentrations; piscivores and carnivores had the highest Hg concentrations and exhibited significant Hg biomagnification. Our results showed that medium- to large-bodied fishes commonly eaten by local people contained Hg values that exceed the World Health Organization (WHO) criteria, and pose a health concern for riverine communities along the Mazaruni River that depend on fish as their main source of protein. Further research to determine the sources of Hg contamination and how it affects human health in this neotropical river must become a top priority. In addition, more research on how Hg contamination impacts the fishes themselves and overall aquatic biodiversity is also needed in the Mazaruni River which has both high fish endemism and diversity.
Keywords: Biomagnification, Gold mining, Guiana Shield, Stable isotopes, Trophic structure.
Na América do Sul, a contaminação por mercúrio devido às operações de mineração de ouro é uma ameaça à biodiversidade e à saúde humana. Nós examinamos as concentrações de mercúrio (Hg) em peixes que constituem importantes pescarias de subsistência em afluentes minerados e não minerados no médio rio Mazaruni, Guiana. As concentrações de mercúrio e a estrutura trófica da teia alimentar (baseada em isótopos estáveis de carbono e nitrogênio) foram caracterizadas para fontes basais primárias e 39 espécies de peixes representando sete guildas tróficas. Os peixes coletados em locais minerados tiveram maiores concentrações de mercúrio; piscívoros e carnívoros tiveram as maiores concentrações de Hg e exibiram biomagnificação significativa de Hg. Nossos resultados mostraram que peixes de corpo médio a grande comumente consumidos pela população local continham valores de Hg que excedem os critérios da Organização Mundial de Saúde (OMS) e representam uma preocupação para a saúde das comunidades ribeirinhas ao longo do rio Mazaruni que dependem dos peixes como sua principal fonte de proteína. Outras pesquisas para determinar as fontes de contaminação por Hg e como isso afeta a saúde humana neste rio neotropical devem se tornar uma prioridade. Além disso, mais pesquisas sobre como a contaminação por Hg impacta os próprios peixes e a biodiversidade aquática em geral também são necessárias no rio Mazaruni, que tem alto endemismo e diversidade de peixes.
Palavras-chave: Biomagnificação, Mineração de ouro, Planalto das Guianas, Isótopos estáveis, Estrutura trófica.
Introduction
Mercury (Hg) is a naturally occurring heavy metal that becomes highly toxic to living organisms when it transforms into the organic form of methylmercury (MeHg) (Sweet, Zelikoff, 2001). Mercury contamination is derived from both natural and anthropogenic sources and has been a major human-health concern in South America since the early 1900s (Veiga, 1997). Natural sources of mercury emissions include forest fires, weathering of soil, and volcanic emission (Pacyna et al., 2010), while the largest sources of anthropogenically-derived Hg in the environment include industrial emissions, deforestation, erosion from agriculture, and artisanal and small-scale gold mining (ASGM). ASGM, in particular, uses liquid elemental mercury (Hg⁰) for gold amalgamation. After the amalgamation process, Hg is released into the atmosphere or deposited as mining waste directly into the soil and water (Telmer, Veiga, 2009). After Hg enters aquatic environments, it can persist there perpetually, and once methylated (MeHg), will bioaccumulate in aquatic food webs, thereby posing a health risk to human populations consuming fish. Controlling Hg contamination in the environment has been an issue of concern for local, national, and international health institutions because predatory fish that are consumed by riverside human populations in these developing countries contain high Hg levels (Veiga, 1997; UNEP, 2013).
The Guiana Shield ecoregion extends across the countries of Guyana, Suriname, French Guiana, Brazil, and Venezuela in northeastern South America and has considerable mineral resources such as gold, diamonds, iron, and bauxite (Hammond, 2005). This ecoregion houses the largest global repository of tropical forest vegetation on Precambrian terrain (Hammond, 2005; Dezécache et al., 2017) and the greatest concentration of global freshwater biodiversity with high levels of endemism (Reis et al., 2003; Alofs et al., 2014). However, this region is threatened by gold mining operations that have severely degraded aquatic habitats via siltation and river channel substrate alteration (Miller et al., 2003). In 2017, gold production in Guyana accounted for 10% of the total GDP (Hilson et al., 2019; Bank of Guyana, 2017), with ASGM accounting for 70% of the country’s gold production (Watson et al., 2020). With the increased global demand for gold and the rising of gold price, mining operations (small-scale and medium-scale) have become more common in other countries within the Guiana Shield causing both environmental and social problems (Hammond et al., 2007; Dezécache et al., 2017; Martinez et al., 2018; Watson et al., 2020). Poorly managed mining operations intensify deforestation, increase sediment loads, and increase Hg accumulation in rivers, wildlife, and people (Cleary, 1990). High Hg concentrations derived from gold mining activities have been found in fluvial sediments of the Essequibo and Mazaruni Rivers in Guyana (Miller et al., 2003; Couture et al., 2005; Howard et al., 2011) and the Cuyuni River (an Essequibo tributary) in Venezuela (Nico, Taphorn 1994). Unsafe levels of Hg in carnivorous fish tissues have been reported from Suriname and French Guiana (Fréry et al., 2001; Ouboter et al., 2012) and in hair samples from indigenous communities in Guyana (Watson et al., 2020). Gold mining alters in-stream habitat structure (Lacerda et al., 2004; Mol, Ouboter, 2004), resulting in habitat transformation that shifts fish diversity and community composition (Miller et al., 2003; Mol, Ouboter, 2004; Barbieri, Gardon, 2009; Brosse et al., 2011). In the Upper Mazaruni River, gold mining is concentrated in the main channel, resulting in high turbidity and sediment accumulation (Alofs et al., 2014). This is particularly concerning because some species endemic to the Mazaruni drainage appear to be highly associated with main channel habitats (López-Fernández et al., 2012).
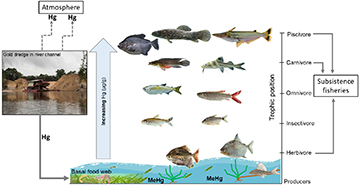
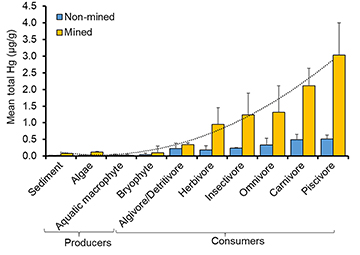
In aquatic ecosystems, food web structure and consumer traits (e.g., body size, trophic ecology) influence the concentration of Hg, in particular MeHg, assimilated and accumulated by organisms (Campbell et al., 2008) (Fig. 1). Primary producers (e.g., phytoplankton and periphyton) concentrate MeHg directly from water (Pickhardt, Fisher, 2007), whereas fish are exposed to MeHg via prey consumption (Driscoll et al., 2007). Globally, fish are recognized as important source of MeHg in humans and considerable efforts are underway to quantify Hg levels in species targeted for subsistence and commercial fisheries. Studies of Hg contamination in aquatic ecosystems in the Neotropics, and in particular, from the Guianas, are still lacking (Fréry et al., 2001; Kwon et al., 2012; Ouboter et al., 2012; Marshall et al., 2016). The seasonal hydrological cycle of tropical rivers is critical for Hg exposure of riverine species. For example, the extension of the streamflow events from river channels into floodplain during the seasonal inundation (Lowe-McConnel, 1987; Winemiller et al., 2014) have the potential to decrease oxygen availability for redox reactions, increasing the microbial conversion rate of Hg to MeHg (Gilmour et al., 1992; Singer et al., 2016). Guimarães et al. (2000) found that floating macrophytes and increased terrestrial detritus during the Amazon River’s inundation pulse are important substrates for mercury methylation by sulfate reducing bacteria because as they decompose, they accumulate MeHg in the water and create direct and indirect pathways for MeHg to aquatic consumers (Roulet et al., 2001).
FIGURE 1 | Conceptual model of how Hg enters riverine food webs of the Mazaruni River, Guyana. First, artisanal gold mining operations installed in the river use a suction dredge to reach gold contained in bottom sediments. These mining operations use mercury for gold amalgamation of which the majority is lost to the atmosphere or river. Once in the river, Hg can be methylated by microbes into the toxic Methylmercury (MeHg) and be assimilated rapidly by aquatic biota. Hg concentration bioaccumulates and biomagnifies in the trophic food chain. Fishes at higher trophic levels [piscivores and carnivores (e.g., piranhas, aimaras, and catfishes)] are important items in the diet of local communities and can become a direct source of Hg uptake via direct consumption.
Tropical fish communities span a range of trophic guilds (e.g., herbivores, carnivores, piscivores) (Fig.1), and occupy virtually all ecological niches (e.g., benthic to pelagic species) (Lowe-McConnell, 1987; Winemiller, 1991; Montaña, Winemiller, 2013). In addition, many species are dependent on seasonal flooding pulses for extensive migrations and reproduction (Jepsen, Winemiller, 2007). Because these fish communities often comprise subsistence riverine fisheries, it is relevant to investigate the potential differences in Hg bioaccumulation of fishes in aquatic food webs. Mercury biomagnification in trophic networks (i.e., cumulative MeHg transfers between the successive consumer levels of the food chain) can lead to high Hg concentrations in piscivorous species (Lavoie et al., 2013; Pouilly et al., 2013) and extensive Hg burdens in the entire fish biomass (Watras et al., 1995). Biomagnification is positively correlated to food chain length (Cabana, Rasmussen, 1994; Chumchal et al., 2011), which can be derived from nitrogen stable isotopes. Nitrogen isotopes provide an estimate of consumer trophic position (Post, 2002). The slope of the relationship between the mean of Hg concentration and trophic position, referred as a food web magnification factor, can be used to compare the transfer efficiencies between MeHg and biomass in food webs (Cabana, Rasmussen, 1994; Jardine et al., 2006). A positive relationship between Hg concentrations and body size has been reported for several piscivorous fish species that are important fisheries across the Guianas such as Hoplias malabaricus (Bloch, 1794), Serrasalmus rhombeus (Linnaeus, 1766), and Plagioscion squamosissimus (Heckel, 1840) (Lacerda, Salomons, 1998; Mol et al., 2001; Maury-Brachet et al., 2020). Unfortunately, fish consumption is considered one of the main pathways of Hg intake by humans in South America (Pacyna et al., 2010). The World Health Organization (1990) established that the safe limit for fish consumption is 0.05 μg Hg g-1 fish. Exposure of local people to Hg of concern because they consume large amounts of fish from local rivers that are contaminated with mercury. Several studies of fishes from neotropical rivers polluted by gold mining activities have already reported Hg concentrations that exceed these international guidelines (Fréry et al., 2001; Mol et al., 2001; Venturieri et al., 2017).
In this study, we quantified total Hg concentrations and biomagnification trends in 39 fish species from the middle Mazaruni River in Guyana to understand the extent of Hg contamination. We examined a range of trophic guilds and fish body sizes the collected in mined and non-mined sites and interpret these results in the context of both the ecosystem and human health. To evaluate how Hg bioaccumulates and biomagnifies through this river system, we used carbon (δ13C) and nitrogen (δ15N) stable isotopes to characterize the vertical trophic position and energy sources of consumers (Fry, 2006). To make inferences between Hg concentrations in fish and the potential risk to human health, we compared total Hg values to the standard values (0.5 μg Hg/g) established for human consumption (WHO, 1990). Our comparisons emphasized primarily fish species that are targeted for local subsistence fisheries (Tab. 1). Mercury concentrations were examined in fish assemblages from sites impacted by gold mining operations and from sites that were semi-natural, where nearby mining operations were not observed. We expected that differences in anthropogenic activities, primarily gold mining, are likely to affect the biological production, food web structure and consequently Hg bioaccumulation of riverine food webs.
Material and methods
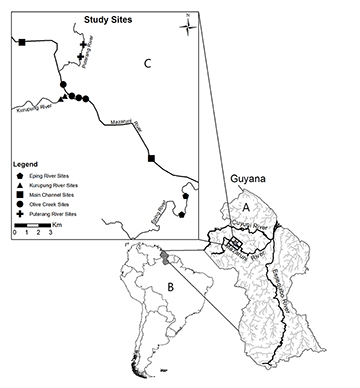
Study area. The Mazaruni River in Guyana is a tributary of the Essequibo River in northern Guyana. The Essequibo River is the largest river in Guyana and known as the major fluvial system connecting to the Amazon River during the rainy season via the Rupununi wetlands of South-Central Guyana (Souza et al., 2020). The Mazaruni River, which is an important source of alluvial gold and diamonds, originates in the western forests of the Pakaraima Mountains and after it descends from the Guiana highlands, it curves northeastward near the town of Bartica, where it joins the Cuyuni River just upstream the confluence with the Essequibo River (Figs. 2A,B). From lowland to upstream reaches, the Mazaruni River changes in water properties, substrate composition and channel geomorphology (Miller et al., 2003). The lowland reaches of the Mazaruni River are characterized by a branching pattern with extensive bedrock exposure creating white water conditions, while the upper drainage reaches contain small pristine black water tributaries that are isolated from the lowland basin by rapids and waterfalls. Our survey sites were located in the middle Mazaruni River drainage (Figs. 2B,C). At this location, the main channel of the Mazaruni River and adjacent tributaries have been highly impacted by gold mining activities, consequently affecting the channel substrate, riparian soils and vegetation, and water turbidity. Our field observations suggest that sites impacted by gold mining activities contain homogeneous substrates (e.g., artificial tailing beaches) likely due to relocation of river substrate by gold dredges, but also high turbidity and high total suspended sediments caused by siltation from mining operations (Fig. 3).
Our surveys were conducted during the dry season of 2018 in mined (Puterang, Kurupung and Mazaruni River at Olive Creek) and non-mined (Eping Creek) river sites in the middle Mazaruni River drainage (Figs. 3A-H). Each site was sampled only once during this season. Eping Creek (06⁰07’29”N 60⁰04’08”W), is a blackwater tributary with low conductivity (range 4.0–13.0 μS/cm), low total suspended solids (TSS) in the water column (0.50 mg/L) and high water transparency (95–130 cm depth measured with Secchi disk). Eping Creek appeared not be mined at the time of our fish surveys. However, our field observation suggests that mining must have been occurred in the past because we observed abandoned mining equipment and a clear reconfiguration of the banks and riparian forest. In comparison with surveys conducted in the Eping Creek in 2016 (Andrade et al., 2019), our observations of the landscape and water conditions did not report major changes in this creek. The riparian forest and its black waters (Figs. 3A,B) appeared less impacted as compared with other mined locations. The Puterang River (06⁰15’14”N 60⁰08’26”W), Kurupung River (06⁰12’36”N 60⁰12’59”W), and main channel of Mazaruni River (06⁰12’53”N 60⁰08’19”W) near Olive Creek were considered mined locations as we observed active mining activities along the main channels. In these three rivers, water conductivity was higher (range 14.2–39.3 μS/cm) while TSS (range 21.0–45.0 mg/L) and water transparency (10–45 cm depth) were lower than in Eping Creek.
Sampling procedures and analysis. Fishes were collected from multiple habitats within each site using multiple fishing gears including seines (6.0 m length x 1.8 m deep, 5-mm mesh), gillnets and hook-and-line. Shrimps were collected with seines. In the field, fishes were euthanized in clove oil, identified to lowest taxonomic levels and measured (standard length, SL in mm) before extracting muscle tissues for Hg and stable isotope analysis. Muscle tissues were taken from the dorsal flank of the fish near the dorsal fin using a sterilized stainless-steel scalpel. Tissue samples for total Hg analysis were placed in individual Ziploc bags and stored in ice until analysis. Tissues for stable isotope analysis were preserved in salt following the methods of Arrington, Winemiller (2002). The primary producers collected included aquatic macrophytes, benthic algae, and bryophytes (moss). Aquatic macrophytes and bryophytes were collected by hand, cut into small pieces, and placed into Ziploc bags for later processing. Sediment samples were collected from the surface of the bottom of the river about 5 cm depth using a Petri dish and spatula and were frozen until analysis. In the laboratory, all samples were soaked and rinsed with distilled water, placed in glass vials, and then dried in an oven at 60°C for 48 h. Once dried, samples were ground to a fine, homogenous powder using a mortar and pestle and then stored in clean glass vials 94°C.
FIGURE 2 | Locations of sampling sites in the middle Mazaruni River drainage in A. Guyana within B. South America. Sampling sites included location in the C. Main channel of the Mazaruni River (mined) and three main tributaries including Eping River (non-mined), Puterang and Kurupung rivers (mined).
FIGURE 3 | Sampling locations on the middle Mazaruni River: A. Main channel of the upper Eping River a small, black water tributary of the Mazaruni River. This river appeared more pristine and less impacted by gold mining activities; B. Sandy, shallow habitat sampled in middle channel of the upper Eping River; C. A gold dredge in the middle of the Kurupung River, on the right side is evidence of soil removal that resulted from mining activities; D. Deforestation (to establish mining stations) along the Kurupung River upstream, left bank from the confluence with the Mazaruni River; E-F. Main channel of the Mazaruni River at Olive Creek. At this location, the main channel of Mazaruni River is highly impacted by gold mining activities and gold dredges and ‘tailing’ beaches are commonly observed; G-H. The main channel of the lower Puterang River before the confluence with the Mazaruni River. At these locations, the Puterang River carries down heavily silted sediments that are spilled into the Mazaruni River.
To examine the influence of energy sources and vertical trophic structure in mined and non-mined river sites, samples were analyzed for stable isotope analysis of carbon (δ13C) and nitrogen (δ15N). Samples of ground fish tissue were weighed (1.2–2.5 mg) into Ultrapure tin capsules, and sent to the Center of Applied Isotope Studies at University of Georgia (Athens, GA, USA) of for analysis using a Delta V mass spectrometer. Isotope ratios were reported in the standard of δ13C and δ15N notation (in parts per thousand ‰) relative to the international standards (atmospheric N2 and PeeDee belemite) (Fry, 2006). Mercury analyses were conducted at the Texas Research Institute for Environmental Studies (TRIES) at Sam Houston State University (Huntsville, Texas, USA). Samples of fish muscle, primary producers and sediments were analyzed for Hg concentrations. Hg concentrations were measured in sediments, where methylation is supposed to occur and delivered to the basal food web (Singer et al., 2016).
All samples for Hg analysis were rinsed with distilled water, placed in glass vials, and then dried in an oven at 60°C for 48 h. Dried samples were homogenized into a fine powder sample and approximately 20 g of each dried sample were used for total Hg analysis. Mercury detection in the samples was determined with the method of cold vapor atomic absorption spectroscopy (CVAAS, Clesceri et al., 1998) using the Millennium Merlin Mercury Analyzer.
All fish species used for stable isotope and total Hg analyses were classified into major trophic guilds (Tab. 1) in the food web using the trophic designations of Richard et al. (2000), Hoeinghaus et al. (2003), and Montaña, Winemiller (2013). Trophic guilds include piscivore, carnivore, omnivore, invertivore, algivore, and algivore/detritivore guilds. In order to depict differences in the isotopic signatures and vertical trophic position of fish species between mined and non-mined sites, we constructed δ13C and δ15N biplots. Values of Hg concentration in sediment, primary producers, and fish (trophic guilds) were compared across surveyed sites and a polynomial regression was performed on total Hg concentration across producers and consumers in the riverine food web to depict overall patterns of Hg biomagnification. In addition, linear regression was performed on total Hg concentration and body size of target fish species known to be used for consumption by local riverine people in the middle Mazaruni River. Differences in δ15N and total Hg among guilds were tested using a Kruskal-Wallis (K-W) test on untransformed data followed by a Mann-Whitney pairwise comparison test. For all tests, type I error was set to α = 0.05.
Results
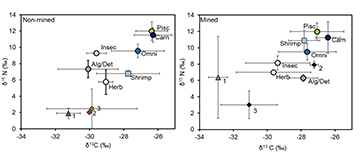
The values of total Hg concentrations and δ13C and δ15N in the fish assemblage from the dry season indicate Hg biomagnification is occurring in a wide range of trophic positions and basal energy sources in both mined and non-mined sites in this river food web (Figs. 4–5). Fish trophic levels spanned primary consumers (herbivores) to tertiary consumers (top predators: carnivores, piscivores) (Fig. 4). Most herbivore, algivore/detritivore and insectivore taxa in non-mined sites had mean δ13C values similar to basal sources such as benthic algae and aquatic macrophytes and when compared to mined site, these values were lower on average (Fig. 4). At mined sites, the δ13C of benthic algae became enriched on average. Thus, it appears that benthic algae could be the main production source supporting higher trophic consumers such as omnivores, carnivores and piscivores. The δ15N values of basal sources also exhibited variation on average between study sites (Fig. 4). Shrimps became δ15N enriched in mined sites when compared to non-mined and bryophyte and benthic algae were about two-fold higher in mined sites (Fig. 4). On average, values of δ15N in consumer fishes were positively related to trophic guilds and δ15N values were slightly more δ15N enriched in carnivores and piscivores in mined sites. Overall, there was only a marginal variation (K-W: H = 4.01, p < 0.05) in the δ15N values between mined and non-mined sites.
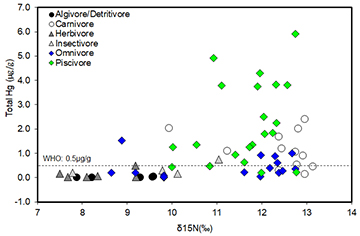
Mean Hg concentrations ranged from 0.02 to 5.92 μg/g among fish species (Tab. 1). Hg concentrations were significantly different among trophic groups between mined and non-mined sites (K-W test, H = 12.7, p < 0.003), with piscivores and carnivores having significantly greater Hg concentrations than the lower trophic guilds. Fishes from mined sites, and in particular, carnivores and piscivores had higher Hg concentration on average (Tab. 1). Regressing Hg concentrations against δ15N values (7.50 to 12.74‰) of all surveyed samples indicated substantial biomagnification of Hg in the Mazaruni River food web (F = 4.40, p < 0.004; Fig. 5). The linear regression was log Hg = -1.19 + 0.16 (δ15N) with a weak strength of the relationship (r2 = 0.13). The Hg concentrations for carnivores and piscivores were higher than any other trophic group, and in many cases, some piscivores showed Hg concentrations that were above the WHO criteria (0.5 μg Hg/g) for fish tissue (Fig. 5). Piscivores such as S. rhombeus and H. malabaricus, which were collected in both mined and non-mined sites had Hg concentrations above 0.5 μgHg/g (Hg ranges between 0.51 – 5.92 μgHg/g). These Hg levels are of special concern because both Piranhas (Serrasalmus) and Houri (Hoplias) along with some catfishes (e.g., Dwalla – Ageneiosus spp.)and Tiger catfish (Pseudoplatystoma fasciatum (Linnaeus, 1766)) and Lukanani (e.g., Cichla spp.) (Tab. 1) are commonly consumed by local communities along the Mazaruni River.
FIGURE 4 | Mean nitrogen (δ15N) and carbon (δ13C) isotope ratios of fish species collected from mined (right panel) and non-mined (left panel) sites in the Mazaruni River, Guyana. Species were grouped together based on their trophic guild. Each symbol represents the average of all species within each trophic guild. Numbers represent the basal resources collected at surveyed sites (1 = bryophyte, 2 = benthic algae, 3 = aquatic macrophytes). The abbreviations for the fish trophic guilds: Algivore/Detritivore (Alg/Det), Insectivore (Insec), Herbivore (Herb), Omnivore (Omni), Carnivore (Carn), Piscivore (Pisc), as well as one shrimp (Macrobrachium).
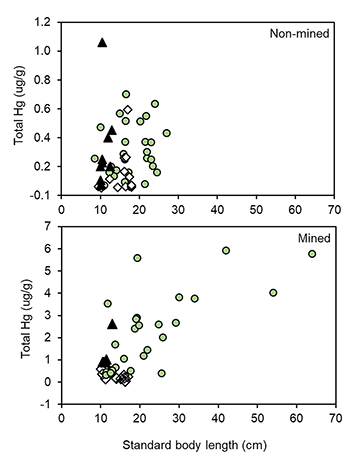
On average, mercury concentrations in sediments (all samples, 0.03 μg/g[SD±0.05]) and basal primary producers (all samples, 0.04 μg/g[±0.11]) were significantly lower when compared to consumers (all samples, 1.87μg/g[±1.20]). When examined by site, there was a trend for increasing Hg concentrations in sediments and benthic algae (mean: 0.07μg/g[±0.01] and 0.12 μg/g[±0.01], respectively) at mined sites (Fig. 6). On average, Hg concentrations appeared to bioaccumulate at the top of the food web with piscivorous fishes containing the highest Hg values in mined sites. The polynomial trendline (y = 0.05x2 – 0.22 + 0.28) also suggested a strong strength of the relationship (R2 = 0.98) (Fig. 6). Among the 39 species examined in this study, several species including piscivores, carnivores and herbivores (i.e., frugivores) are targeted by local fishermen for food consumption. Overall, Hg concentrations did not appear to correlate with body size in herbivorous fish (e.g., Myloplus asterias) (F = 2.16, p = 0.14, r2 = 0.03; Fig. 7). However, in carnivores and piscivores, we found a significant (F = 38.8, p < 0.003, r2 = 0.40; Fig. 7), positive relationship between increasing consumer body size and Hg concentration in fish tissue.
TABLE 1 | Fish species and their mean mercury concentrations (µg Hg/g, dry weight) from mined or non-mined sites. Species were classified to trophic guilds based on published literature. N indicates total number of samples analyzed.
| Scientific name | Trophic guild | Use for consumption | N | Mined (mean THg) | Non-mined (mean THg) |
| Acestrorhynchus microlepis | Piscivore | N | 2 | 2.37 | |
| Ageneiosus inermis | Piscivore | Y | 5 | 3.24 | |
| Ageneiosus ucayalensis | Piscivore | Y | 1 | 5.92 | |
| Auchenipterus nuchalis | Piscivore | Y | 1 | 3.01 | |
| Boulengerella cuvieri | Piscivore | Y | 3 | 4.29 | |
| Cynodon septenarius | Piscivore | Y | 5 | 4.41 | 0.51 |
| Cichla ocellaris | Piscivore | Y | 2 | 0.85 | |
| Hoplias malabaricus | Piscivore | Y | 3 | 2.68 | 0.77 |
| Hydrolycus armatus | Piscivore | Y | 4 | 2.15 | 0.55 |
| Serrasalmus rhombeus | Piscivore | Y | 59 | 1.52 | 0.32 |
| Serrasalmus eigenmanni | Piscivore | Y | 14 | 1.01 | 0.15 |
| Trachycorystes trachycorystes | Piscivore | N | 4 | 0.51 | 0.4 |
| Pseudoplatystoma fasciatus | Piscivore | Y | 1 | 2.78 | |
| Charax gibbosus | Carnivore | N | 6 | 4.21 | 0.42 |
| Crenicichla lugubris | Carnivore | N | 3 | 2.22 | 0.58 |
| Pimelodella sp. | Carnivore | N | 3 | 1.05 | 0.46 |
| Pimelodella gergi | Carnivore | N | 1 | 1.01 | |
| Hemisorubim platyrhynchos | Carnivore | Y | 2 | 1.7 | 0.45 |
| Pachypops fourcroi | Carnivore | Y | 1 | 0.4 | |
| Pseudopimelodus bufonius | Carnivore | Y | 3 | 1.33 | 0.54 |
| Anostomus anostomus | Omnivore | N | 5 | 1.08 | 0.31 |
| Brycon pesu | Omnivore | N | 6 | 2.68 | 0.32 |
| Bryconops melanurus | Omnivore | N | 6 | 1.21 | 0.59 |
| Caenotropus labyrinthicus | Omnivore | N | 2 | 0.75 | |
| Chalceus macrolepidotus | Omnivore | N | 3 | 2.6 | 0.59 |
| Cichlasoma bimaculatum | Omnivore | Y | 3 | 2.56 | 0.24 |
| Doras carinatus | Omnivore | N | 3 | 0.47 | |
| Leporinus agassizi | Omnivore | N | 2 | 0.31 | |
| Triportheus albus | Omnivore | Y | 8 | 2.32 | 0.37 |
| Biotodoma cupido | Insectivore | N | 3 | 2.01 | |
| Geophagus n.sp. | Insectivore | N | 2 | 0.23 | |
| Jupiaba polylepis | Insectivore | N | 9 | 1.12 | 0.25 |
| Brycon amazonicus | Herbivore | Y | 1 | 0.02 | |
| Myloplus asterias | Herbivore | Y | 6 | 0.19 | 0.06 |
| Myloplus rubripinnis | Herbivore | Y | 2 | 0.15 | |
| Curimata ocellata | Algivore/Detritivore | N | 4 | 0.085 | 0.035 |
| Hypostomus sp. | Algivore/Detritivore | N | 6 | 0.51 | 0.34 |
| Parodon sp. | Algivore/Detritivore | N | 5 | 0.06 | |
| Platydoras hancocki | Algivore/Detritivore | N | 4 | 0.31 |
FIGURE 5 | Biomagnification of Hg in the Mazaruni River, Guyana, represented as the log total Hg concentrations plotted against nitrogen (δ15N) isotope ratios. Species were grouped together based on their trophic guild. Each symbol represents the average of all species within each trophic guild. The dashed horizontal line indicates the reference limit of 0.5 µg/g by WHO guideline for Hg in fish consumed by humans.
FIGURE 6 | Mean Hg concentrations (dry weight) in sediments and instream biota separated by trophic groups (producers and consumers) between mined and non-mined sites in the Mazaruni River, Guyana. Species were grouped together based on their trophic guild. Polynomial trendline: y = 0.05×2 – 0.22x + 0.28, coefficient of determination R² = 0.98.
FIGURE 7 | Relationships between body size (standard length, mm) and total Hg of fishes commonly used for consumption. Top panel (non-mined) contains three species that were common at mined sites (bottom panel). Relationships were estimated for five species that are commonly found and consumed, and for what a class structure was observed. Symbols represent individual fish. Clear diamonds = frugivores [1 sp.: Myloplus asterias (n = 19)], dark triangles = carnivores [1 sp.: Serrasalmus eigenmanni (n = 14)], green circles = piscivores [(3 spp.: S. rhombeus (n = 24), Ageneiosus ucayalensis (n = 2), Hoplias malabaricus (n = 1)].
Discussion
Our findings confirm widespread Hg contamination in aquatic food webs of the middle Mazaruni River due to increased ASGM activities. In this specific region, a large number of gold dredges have been installed in the main channel of the Mazaruni River with observable consequences in the river water quality such as high turbidity, high concentration of suspended sediments in the water column, and presence of “tailing” beaches which have resulted from mining operations. The positive relationship between the vertical food web structure (i.e., estimated by δ15N of fishes) and the concentrations of Hg found in fish tissues suggest that mercury biomagnification is occurring in the aquatic food web of the Mazaruni River. Fish Hg contamination appears linked to biomagnification enhanced by benthic algae and primary consumers (e.g., shrimps, the main invertebrate analyzed) contribution to the food web and probably in combination with the altered river conditions that perhaps facilitate methylation in this floodplain river.
Studies in other temperate and tropical aquatic systems suggest that Hg biomagnification trends based on fish-only food webs normally fall between 0.2 and 0.3 (Campbell et al., 2003, 2005; Pouilly et al., 2013). Our estimated slope of 0.22 suggests that Hg bioaccumulation rates in the Mazaruni River food webs are as high as those observed in other Neotropical rivers (Pouilly et al., 2013). Although studies in freshwater ecosystems have demonstrated a positive relationship between Hg biomagnification rates and food chain length (Cabana, Rasmussen, 1994; Molina et al., 2010), the strength of trophic biomagnification appears to be affected by the productivity of the river system (Walters et al., 2015). For instance, in productive systems with abundant algae, Hg bioaccumulation appears to decline via the mechanism of bloom dilution (MeHg burden per cell decreases in algal bloom; Tsui et al., 2010; Walters et al., 2015). The high Hg bioaccumulation that we may be observing could be as a result that high turbidity levels and high amounts of suspended sediments in the water column, due to increased ASGM activities along the Mazaruni River, have consequently reduced the accumulation and distribution of algae in this system due to light limitation. It is important to highlight that in tropical systems, δ15N fractionation is low in trophic transfers compared to other ecosystems (Kilham et al., 2009) perhaps due to the rapid turnover rate of the tissues (McIntyre, Flecker, 2006), therefore, in this context of Hg biomagnification, one should expect increase of Hg per unit change of δ15N. When examining the middle Mazaruni River food web, our results appear to support this prediction, as Hg concentrations in consumer tissues were positively correlated with their trophic position in the food web.
Fish trophic position (measured by enrichment of δ15N ratios) is an important variable to consider in Hg studies because it influences dietary exposure to MeHg. In the Mazaruni River section we studied, carnivores and piscivores exhibited the highest Hg concentrations. Even fishes from these two trophic groups that were collected from non-mined sites showed Hg values above the WHO criteria. As Hg bioaccumulates, it is possible that some fish from non-mined sites still retain high Hg concentrations in their tissues from past mining activities. Although little information is known about mercury deposition, bioaccumulation, and speciation in sediments of the Mazaruni River, one would expect that mining in any particular area of the Mazaruni could contaminate the entire river drainage. For instance, Hg can get buried in sediments for long periods of time. Also, sediments can spread downstream, carrying Hg with them, to all main channel substrate downstream. Migratory species or those that undergo long-distance movements amongst different sites could influence these observed results as well. For instance, some of the large piscivorous catfishes and predatory species like Hoplias, Hydrolycus, and Serrasalmus (Fig. 1) are believed to have seasonal migrations associated with reproduction events (Goulding et al., 2003; Van der Sleen, Albert, 2018). During these migrations, fishes could assimilate Hg from more contaminated locations along the Mazaruni River and tributaries and then migrate into our study sites. Because of widespread of ASGM activities in the Mazaruni River and its tributaries and placer mine introduction of inorganic Hg into these aquatic ecosystems, there is high potential for inorganic Hg in sediments to be transformed by methylation microbes (e.g., sulfate-and iron-reducing bacteria) into bioaccumulative MeHg (Donovan et al., 2016; Singer et al., 2016), consequently increasing the likelihood of MeHg entering the aquatic food webs. Our results of total Hg concentrations in sediments from mined sites roughly yielded similar concentrations of Hg (0.077 μgHg/g) as documented by Miller et al. (2003) in a reach of 350 km along the Mazaruni River. Likewise, in their extensive study of Hg contamination of alluvial sediments within the Essequibo (160 km river reach) and Mazaruni River basins, Miller et al. (2003) showed that high Hg accumulation in alluvial deposits was related to anthropogenic activities such as gold mining.
Although many factors may play a role in Hg transformation into MeHg in riverine sediments, clearly tropical floodplain rivers can represent ideal systems for Hg methylation. This is due to their seasonal connectivity between the main channel and floodplains (i.e., flood pulse; Junk et al., 1989), which can alter the redox conditions and thereby facilitate the microbial conversion of inorganic Hg to the organic form-MeHg (Gilmour et al., 1992; Benoit et al., 1999). However, Hg can enter the river systems via atmospheric deposition as approximately 55% of the mercury used in gold mining operations is assumed to be lost to the atmosphere (Pfeiffer, Lacerda, 1988). Although unlikely, this should be interpreted with caution, high Hg levels in fishes from non-mined sites in Mazaruni River could be attributed to atmospheric transportation and deposition in sediments. While working on pristine and gold mined impacted streams in Suriname, a country that borders Guyana to the east, Ouboter et al. (2012) found that sediment samples and fishes from pristine areas had high levels of Hg concentrations comparable to mined areas. Their main conclusion is explained by the fact that atmospheric transportation of Hg from adjacent mining areas is deposited in pristine areas where Hg can be freely available for methylation.
We expected that differences in water quality due to mining impacts would affect trophic structure since these patterns have been observed in rivers in Suriname and Bolivia (Ouboter et al., 2012; Pouilly et al., 2013). In regards to δ15C, values of basal resources such as bryophytes, benthic algae, and aquatic macrophytes were more δ13C depleted on average, with the exception of benthic algaeat mined sites that showed enriched δ13C values. Lower δ15C values in tropical (Sanseverino et al., 2012) and temperate (Bastviken et al., 2003) freshwater ecosystems have been associated with methane production from anoxic sediments during flooding events. Wantzen et al. (2002) suggested that the seasonal flood pulse in the Brazilian Pantanal affect variations of the methane production resulting in low δ15C values, which was also reflected in herbivore fishes. We did not eliminate the possibility of methane production in the Mazaruni River due to increased sedimentation attributed to gold mining activities and deforestation (Miller et al., 2003; Alofs et al., 2014), although our lower δ13C values could tentatively be interpreted as an effect of high methane production by methanotrophic bacteria further research will be necessary. The δ13C values for trophic consumers also appeared to vary between sites. For example, in mined sites, more consumers appear aligned with δ13C originating from benthic algae sources that may explain a consistent relationship between high Hg values for benthic algae and primary consumers at mined sites. Benthic algae along with aquatic macrophytes, and riparian trees are dominant energy sources in tropical food webs source (Roach, 2013; Roach et al., 2014; Montaña et al., 2020). Therefore, the link between δ13 C and Hg concentrations in fishes at mined sites could reflect that methylation is occurring at the bottom of the food web, where perhaps sediment accumulation and low productivity reinforce Hg methylation and transportation to higher trophic levels. Unfortunately, information about bioavailable Hg in tropical rivers is still lacking. Roulet et al. (2001) suggested that in large floodplain rivers, MeHg is associated with allochthonous sources and therefore fishes feeding in allochthonous materials may enhance Hg assimilation. Although we did not measure either Hg or stable isotopes in allochthonous sources (e.g., riparian plants), we are aware that aquatic macrophytes may contribute to the energy source of herbivorous fishes (Roach et al., 2014). There was not a consistent relationship with Hg concentration, as herbivores were lower in the food web.
While Neotropical freshwater fishes are among the most diverse taxa on the planet, they have been increasingly impacted by humans due to the reliance of societies on freshwater ecosystem services and the lack of sustainable practices and conservation policies (Pelicice et al., 2017). For decades, most countries within the Guiana Shield including Guyana, Suriname and French Guiana, have economically relied on artisanal and small-scale gold mining (ASGM) activities. The widespread use of Hg in gold mining activities has driven contamination of Guianan river ecosystems including fishes and humans, threatened ecosystems and biodiversity, human health and livelihoods of local riverine communities (Hammond et al., 2007; Ouboter et al., 2012; Maury-Brachet et al., 2020; Watson et al., 2020). Unsafe Hg concentrations have been reported in carnivorous fishes and human hair samples from the lower Mazaruni River (Singh et al., 2001; Watson et al., 2020). Watson et al. (2020) also reported high mercury levels in hair samples for residents living close to ASGM activities in the southern Rupununi region in Guyana. From our findings, it is noticeable that fish trophic guild exhibited a clear relationship with Hg levels, which helps to provide information about the trophic groups that can be safety for human consumption. Most of the piscivorous species analyzed in this study including Ageneiosus spp., H. malabaricus, S. rhombeus, Cichla ocellaris Bloch & Schneider, 1801, and Hydrolycus armatus (Jardine, 1841), are commonly used by indigenous people (i.e., Amerindians) in the Mazaruni for food protein. Exceptionally high Hg concentrations were observed in these species, and these patterns are consistent with studies in other rivers within the Guianas (for example: Mol et al., 2001 (Suriname); Richard et al., 2000; Fréry et al., 2001; Ouboter et al., 2012; and Maury-Brachet et al., 2020 (French Guiana) and Amazon basin (Roach et al., 2013; Venturieri et al., 2017).
Our findings have important implications for fisheries conservation and human health in the Mazaruni Region. First, the Mazaruni River drainage is recognized for having high fish diversity and endemism (Albert, Reis, 2011; Reis, 2013), yet this ichthyological biodiversity is poorly studied and threatened by gold mining operations. Second, fishing provides a major source of protein for indigenous communities in Guyana (Couture et al., 2005; Watson et al., 2020). Watson et al. (2020) reported that more than 50% of all participants from four communities in South Rupununi, Guyana, consumed fish daily and that indigenous people living close to the ASGM activities and consuming fish daily showed the highest concentrations of mercury in their hair samples. From our personal observations during field surveys along the Mazaruni, there are certainly many families that consume fish, more than once per week. This situation may pose a health risk to people because many of the fish species analyzed from Mazaruni River had high concentrations of mercury that exceeds the WHO recommended criteria. Thus, assuming that 95% of the total Hg present in fish tissues was MeHg (Bloom, 1992), MeHg concentrations reported for most piscivores, carnivores and some omnivores were above the WHO tissue criteria for consumption. Among the nineteen species commonly reported for consumption by local people (Tab. 1), a positive relationship between body size and Hg bioaccumulation was observed for piscivorous species with a large size structure (e.g., S. rhombeus) indicating the certain trophic guilds (e.g., piscivores) and long-lived fish can have elevated Hg concentrations that can be passed on to humans via consumption. As expected, our results found support for patterns between trophic guilds and Hg concentrations reported for many tropical and temperate aquatic systems, with larger piscivores typically having higher Hg concentrations due to biomagnification through the food web. Our results are critical for informing how trophic position and fish size are factors that can be easily identified by riverine people in Guyana who consume fish regularly and could be used to help them to minimize the intake of fishes that are likely to contain high Hg concentrations.
Although our results are from a single season, they suggest that mercury contamination due to gold mining activities may be responsible for the loss of Neotropical freshwater fish diversity. Hg contaminated habitats were also highly turbid resulting in lower quality habitats that reduced fish diversity and shifted in community structure. Hg exposure can have inhibitory effects on fish reproduction such as spawning behavior, fertilization success, and fecundity (Depew et al., 2012). This is particularly concerning for fish species that are very sensitive to changes in habitat and water quality. In the Mazaruni basin, for example, some species of catfish (Rhamdia) and undescribed hypopomid knifefish appear primarily associated with undisturbed river habitats (Alofs et al., 2014). Likewise, high concentrations of Hg bioaccumulation can alter the immune system and disrupt the metabolic processes in fishes, particularly those species feeding at higher trophic levels (Morcillo et al., 2017). Loss or reduction of certain functional groups (e.g., carnivores and piscivores) following high Hg toxicity not only impact the ecological functions of this aquatic ecosystem, but the fisheries (e.g., food security, market value, yields) and other ecosystem services.
In light of the importance of subsistence fisheries in the Mazaruni River, high Hg concentration in fishes and biomagnification rate, we issue a warning to local inhabitants about the health risk of regularly eating large amounts of fish captured from this region. Also, our results agree with other studies conducted in other rivers within the Guiana Shield, which have demonstrated alarming concentrations of mercury accumulation in fish. Accumulation of Hg levels in Indigenous Guyanese populations needs more comprehensive assessments of Hg sources to better inform the potential health effects, both short- and long-term effects, from fish consumption.
While the importance of Hg contamination through fish uptake in humans is well established. The impacts of Hg contamination in Neotropical fish and overall aquatic biodiversity needs further investigations. In Guyana, the ASGM operations have increased in recent decades due to the rising gold price, and along with these operations, greater deforestation and river dredging have been documented (Hammond et al., 2007; Alofs et al., 2014). Experimental studies have shown that high levels of mercury cause behavioral, hormonal, and reproductive changes in birds and mammals (Scheuhammer et al., 2007), but very little is known about the adverse impacts of Hg in fish behavior, gonadal development, reproduction, or even their biomass and diversity. Water turbidity and accumulation of sediments on the main channel of the Mazaruni River are noticeable, which could create hostile living habitats for fishes and affect their biomass as well. Of particular concern about mercury contamination in the Mazaruni River and other rivers in Guyana (e.g., Rupununi and Essequibo) is that the health of these ecosystems is vital for the persistence of biological and cultural communities. High fish endemism has been reported for Mazaruni drainage, but very little is known about how mercury derived from gold mining is impacting these species that appear to be primarily habitat specialists. Although work in other rivers of the Guiana Shield reported effects of ASGM operations on fish communities (Brosse et al., 2011), we recommend more detailed medium-long term studies for monitoring the fish communities in Mazaruni River; and in particular, studies related to changes in fish biomass, diversity, and fish health and reproduction are needed to evaluate the effect of mercury contamination in freshwater biota.
Acknowledgments
We thank Clay Laughrey, Sherica Isaacs, Ravindra Mohandeo, Andre Lyttle, Keith Joris for assistance in the field, Jake Swanson for making the map, and Fiedrich W. Keppeler for translation of the abstract in Portuguese. We thank the Guyana Geological and Mines Commission (GGMC) at Olive Creek for providing lodging during the expedition and The University of Guyana for providing logistic support. The Staff of the Centre for the Study of Biological Diversity (CSBD) at the University of Guyana for the verification process of the fish and EPA-Guyana for processing the research and export permits. This study was conducted under EPA-Guyana permit No 031516 BR003 to EL, CGM and DCT.
References
Albert JS, Reis R, editors. Historical biogeography of Neotropical freshwater fishes. Berkeley: University of California Press; 2011.
Alofs KM, Liverpool EA, Taphorn DC, Bernard CR, López-Fernández H. Mind the (information) gap: the importance of exploration and discovery for assessing conservation priorities for freshwater fish. Divers Distrib. 2014; 20(1):107–13. https://doi.org/10.1111/ddi.12127
Andrade MC, Lopez-Fernandez H, Liverpool EA. New Myloplus from Essequibo River basin, Guyana, with discussion on the taxonomic status of Myleus pacu (Characiformes: Serrasalmidae). Neotrop Ichthyol. 2019; 14(4):e190026. https://doi.org/10.1590/1982-0224-20190026
Arrington DA, Winemiller KO. Preservation effects on stable isotope analysis of fish muscle. Trans Am Fish Soc. 2002; 131(2):337–42. Bank of Guyana. Annual Report 2017 [Internet]. Georgetown; 2017. Available from: https://www.bankofguyana.org.gy/bog/images/research/Reports/ANNREP2017.pdf
Barbieri FL, Gardon J. Hair mercury levels in Amazonian populations: spatial distribution and trends. Int J Health Geogr. 2009; 8(71):1–20. https://doi.org/10.1186/1476-072X-8-71
Bastviken D, Ejlertsson J, Sundh I, Tranvik L. Methane as a source of carbon and energy for lake pelagic food webs. Ecology. 2003; 84(4):969–81. https://doi.org/10.1890/0012-9658(2003)084[0969:MAASOC]2.0.CO;2
Benoit JM, Mason RP, Gilmour CC. Estimation of mercury-sulfide speciation in sediments pore waters using octanol-water partitioning and implications for availability to methylating bacteria. Environ Toxicol Chem. 1999; 18(10):2138–41. https://doi.org/10.1002/etc.5620181004
Bloom NS. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci. 1992;49(5):1010–17. https://doi.org/10.1139/f92-113
Brosse S, Grenouillet G, Gevrey M, Khazraie K, Tudesque L. Small-scale gold mining erodes fish assemblage structure in small neotropical streams. Biodivers Conserv. 2011; 20:1013–26. https://doi.org/10.1007/s10531-011-0011-6
Cabana G, Rasmussen JB. Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature. 1994; 372:255–57. https://doi.org/10.1038/372255a0
Campbell LM, Hecky RE, Nyaundi J, Muggide R, Dixon DG. Distribution and food-web transfer of mercury in Napoleon and Winam Gulfs, Lake Victoria, East Africa. J Great Lakes Res. 2003;29(Suppl 2):267–82. https://doi.org/10.1016/S0380-1330(03)70554-1
Campbell LM, Norstrom RJ, Hobson KA, Muir DCG, Backus S, Fisk AT. Mercury and other trace elements in a pelagic Arctic marine food web (Northwater Polynya, Baffin Bay). Sci Total Environ. 2005; 351–352:247–63. https://doi.org/10.1016/j.scitotenv.2005.02.043
Campbell LM, Verburg P, Dixon DG, Hecky RE. Mercury biomagnification in the food web of Lake Tanganyika (Tanzania, East Africa). Sci Total Environ. 2008;402(2–3):184–91. https://doi.org/10.1016/j.scitotenv.2008.04.017
Chumchal MM, Rainwater TR, Osborn SC, Roberts AP, Abel MT, Cobb GP et al. Mercury speciation and biomagnification in the food web of Caddo Lake, Texas and Louisiana, USA, a subtropical freshwater ecosystem. Environ Toxicol Chem. 2011; 30(5):1153–62. https://doi.org/10.1002/etc.477
Cleary D. Anatomy of the Amazon gold rush. Iowa: University of Iowa Press; 1990.
Clesceri LS, Greenberg AE, Eaton AD. Standard methods for the examination of water and waste water. Washington: American Public Health Association; 1998.
Couture R, Lafleur C, Lambert J. Path of mercury in the environment of alluvial gold mining in pristine areas in Guyana. In: Guyana Environmental Capacity Development Project. Georgetown: Guyana Geology and Mines Commission; 2005.
Depew DC, Basu N, Burgess NM, Campbell LM, Devlin EW, Drevnik PE et al. Toxicity of dietary methylmercury to fish: derivation of ecologically meaningful threshold concentrations. Environ Toxicol Chem. 2012; 31(7):1536–47. https://doi.org/10.1002/etc.1859
Dezécache C, Faure E, Gond V, Salles JM, Vieilledent G, Hérault B. Gold-rush in a forested El Dorado: deforestation leakages and the need for regional cooperation. Environ Res Lett. 2017; 12(3):034013. https://doi.org/10.1088/1748-9326/aa6082
Donovan PM, Blum JD, Singer MB, Marvin-DiPasquale M, Tsui MT. Isotopic composition of inorganic mercury and methylmercury downstream of a historical gold mining region. Environ Sci Technol. 2016;50(4):1691–702. https://doi.org/10.1021/acs.est.5b04413
Driscoll CT, Han Y-J, Chen CY, Evers DC, Lambert KF, Holsen TM et al. Mercury contamination in forest and freshwater ecosystems in the northeastern United States. BioScience. 2007; 57(1):17–28. https://doi.org/10.1641/B570106
Fréry N, Maury-Brachet R, Maillot E, Deheeger M, De Mérona B, Boudou A. Gold-mining activities and mercury contamination of native Amerindian communities in French Guiana: Key role of fish in dietary uptake. Environ Health Perspect. 2001; 109(5):449–56. https://doi.org/10.1289/ehp.109-1240303
Fry B. Stable isotope ecology. New York: Springer; 2006.
Gilmour CC, Henry EA, Mitchell R. Sulfate stimulation of mercury methylation in freshwater sediments. Environ Sci Technol. 1992; 26(11):2281–87. https://doi.org/10.1021/es00035a029
Goulding M, Barthem R, Cañas C, Forsberg B, Ortega H. Amazon Headwaters: wildlife, and conservation in southeastern Peru. Lima: Asociación Para la Conservacióne la Cuenca Amazónica and Amazon Conservation Association; 2003.
Guimarães JRD, Meili M, Hylander LD, Silva EC, Roulet M, Mauro JBN, Lemos RA. Mercury net methylation in five tropical flood plain regions of Brazil: high in the root zone of floating macrophyte mats but low in surface sediments and flooded soils. Sci Total Environ. 2000;261(1–3):99–107. https://doi.org/10.1016/S0048-9697(00)00628-8
Hammond DS. Socio-economic aspects of Guiana Shield forest use. In: Hammond DS, editor. Tropical forests of the Guiana Shield: ancient forests in a Modern world. Cambridge: CABI Publishing; 2005. p.381–480.
Hammond DS, Gond V, De Thoisy B, Forget PM, DeDijn BP. Causes and consequences of a tropical forest gold rush in the Guiana Shield, South America. AMBIO. 2007; 36(8):661–70. Available from: https://www.jstor.org/stable/25547834
Hilson G, McQuilken J, Perks R. State of the artisanal and small scale mining sector. Washington: World Bank; 2019. Available from: https://delvedatabase.org/resources/state-of-the-artisanal-and-small-scale-mining-sector
Hoeinghaus DJ, Layman CA, Arrington DA, Winemiller KO. Spatiotemporal variation in fish assemblage structure in tropical floodplain creeks. Environ Biol Fishes. 2003; 67:379–87. https://doi.org/10.1023/A:1025818721158
Howard J, Trotz MA, Thomas K, Omisca E, Chiu HT, Halfhide T et al. Total mercury loadings in sediment from gold mining and conservation areas in Guyana. Environ Monit Assess. 2011; 179:555–73. https://doi.org/10.1007/s10661-010-1762-3
Jardine TD, Kidd KA, Fisk AT. Applications, considerations, and sources of uncertainty when using stable isotope analysis in ecotoxicology. Environ Sci Technol. 2006; 40(24):7501–11. https://doi.org/10.1021/es061263h
Jepsen DB, Winemiller KO. Basin geochemistry and isotopic ratios of fishes and basal production sources in four Neotropical rivers. Ecol Freshw Fish. 2007; 16(3):267–81. https://doi.org/10.1111/j.1600-0633.2006.00218.x
Junk WJ, Bayley PB, Sparks RE. The flood-pulse concept in river-floodplain systems. In: Doge DP, editor. Proceedings of the international large river symposium. Ottawa: Canadian Journal of Fisheries and Aquatic Sciences Special Publication; 1989. p.110–27.
Kilham SS, Hunte-Brown M, Verburg P, Pringle CM, Whiles MR, Lips KR et al. Challenges for interpreting stable isotope fractionation of carbon and nitrogen in tropical aquatic ecosystems. Verh Int Ver Theor Angew Limnol. 2009; 30(5):749–53. https://doi.org/10.1080/03680770.2009.11902231
Kwon SY, McIntyre PB, Flecker AS, Campbell LM. Mercury biomagnification in the food web of a neotropical stream. Sci Total Environ. 2012; 417–418:92–97. https://doi.org/10.1016/j.scitotenv.2011.11.060
Lacerda LD, Salomons W. Mercury from gold and silver mining: A chemical time bomb? Berlin: Springer Verlag; 1998.
Lacerda LD, Souza M, Ribeiro MG. The effects of land use change on mercury distribution in soils of Alta Floresta, Southern Amazon. Environ Pollut. 2004; 129(2):247–55. https://doi.org/10.1016/j.envpol.2003.10.013
Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, Campbell LM. Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environ Sci Technol. 2013;47(23):13385–94. https://doi.org/10.1021/es403103t
López-Fernández H, Taphorn DC, Liverpool EA. Phylogenetic diagnosis and expanded description of the genus Mazarunia Kullander, 1990 (Teleostei: Cichlidae) from the upper Mazaruni River, Guyana, with description of two new species. Neotrop Ichthyol. 2012; 10(3):465–86. https://doi.org/10.1590/S1679-62252012000300001
Lowe-McConnell RH. Ecological studies in tropical fish communities. Cambridge: Cambridge University Press; 1987.
Marshall BG, Forsberg BR, Thomé-Souza M, Peleja R, Moreira MZ, Freitas CE. Evidence of mercury biomagnification in the food chain of the cardinal tetra Paracheirodon axelrodi (Osteichtyes: Characidae) in the Rio Negro, central Amazon, Brazil. J Fish Biol. 2016; 89(1):220–40. https://doi.org/10.1111/jfb.12952
Martinez G, McCord SA, Driscoll CT, Todorova S, Wu S, Araújo JF, Vega CM, Fernandez LE. Mercury contamination in riverine sediments and fish associated with artisanal and small-scale gold mining in Madre de Dios, Peru. Int J Environ Res Public Health. 2018; 15(8):1584. https://doi.org/10.3390/ijerph15081584
Maury-Brachet R, Gentes S, Dassié EP, Feurtet-Mazel A, Vigorourox R, Laperche V et al. Mercury contamination levels in the bioindicator piscivorous fish Hoplias aimara in French Guiana rivers: mapping for risk assessment. Environ Sci Pollut Res Int. 2020; 27:3624–36. https://doi.org/10.1007/s11356-018-3983-x
McIntyre PB, Flecker AS. Rapid turnover of tissue nitrogen of primary consumers in tropical freshwaters. Oecologia. 2006; 148:12–21. https://doi.org/10.1007/s00442-005-0354-3
Miller JR, Lechler PJ, Bridge G. Mercury contamination of alluvial sediments within the Essequibo and Mazaruni river basins, Guyana. Water Air Soil Pollut. 2003; 148:139–66. https://doi.org/10.1023/A:1025465800121
Mol JH, Ouboter PE. Downstream effects of erosion from small-scale gold mining on the instream habitat and fish community of a small neotropical rainforest stream. Conserv Biol. 2004; 18(1):201–14. https://doi.org/10.1111/j.1523-1739.2004.00080.x
Mol JH, Ramlal JS, Lietar C, Verloo M. Mercury contamination in freshwater, estuarine, and marine fishes in relation to small-scale gold mining in Suriname, South America. Environ Res. 2001; 86(2):183–97. https://doi.org/10.1006/enrs.2001.4256
Molina CI, Gibon FM, Duprey JL, Dominguez E, Guimarães JRD, Roulet M. Transfer of mercury and methylmercury along macroinvertebrate food chains in a floodplain lake of the Beni River, Bolivian Amazonia. Sci Total Environ. 2010; 408(16):3382–91. https://doi.org/10.1016/j.scitotenv.2010.04.019
Montaña CG, Winemiller KO. Evolutionary convergence in Neotropical cichlids and Nearctic centrarchids: evidence from morphology, diet, and stable isotope analysis. Biol J Linn Soc Lond. 2013; 109(1):146–64. https://doi.org/10.1111/bij.12021
Montaña CG, Ou C, Keppeler FW, Winemiller KO. Functional and trophic diversity of fishes in the Mekong-3S river system: comparison of morphological and isotopic patterns. Environ Biol Fishes. 2020; 103:185–200. https://doi.org/10.1007/s10641-020-00947-y
Morcillo P, Esteban MA, Cuesta A. Mercury and its toxic effects on fish. AIMS Environ Sci. 2017; 4(3):386–402. https://doi.org/10.3934/environsci.2017.3.386
Nico LG, Taphorn DC. Mercury in fish from gold-minig regions in the upper Cuyuni River system, Venezuela. Fresenius Environ Bull. 1994; 3(5):287–92.
Ouboter PE, Landburg GA, Quik JHM, Mol JHA, van der Lugt F. Mercury levels in pristine and gold mining impacted aquatic ecosystems of Suriname, South America. AMBIO 2012; 41:873–82. https://doi.org/10.1007/s13280-012-0299-9
Pacyna EG, Pacyna JM, Sundseth K, Munthe J, Kindbom K, Wilson S et al. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos Environ. 2010; 44(20):2487–99. https://doi.org/10.1016/j.atmosenv.2009.06.009
Pelicice FM, Azevedo-Santos VM, Vitule JRS, Orsi ML, Lima DP, Jr., Magalhães ALB et al. Neotropical freshwater fishes imperilled by unsustainable policies. Fish Fish (Oxf). 2017; 18(6):1119–33. https://doi.org/10.1111/faf.12228
Pfeiffer WC, Lacerda LD. Mercury inputs into the Amazon region, Brazil. Environ Technol. 1988; 9(4):325–30. https://doi.org/10.1080/09593338809384573
Pickhardt PC, Fisher NS. Accumulation of inorganic and methylmercury by freshwater phytoplankton in two contrasting water bodies. Environ Sci Technol. 2007; 41(1):125–31. https://doi.org/10.1021/es060966w
Post DM. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002; 83(3):703–18. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Pouilly M, Rejas D, Pérez T, Duprey JL, Molina CI, Hubas C, Guimarães JRD. Trophic structure and mercury biomagnification in tropical fish assemblages, Iténez River, Bolivia. PloS One. 2013; 8(5):e65054. https://doi.org/10.1371/journal.pone.0065054
Reis RE. Conserving the freshwater fishes of South America. Int Zoo Yearb. 2013; 47(1):65–70. https://doi.org/10.1111/izy.12000
Reis RE, Kullander SO, Ferraris Jr CJ. Checklist of the freshwater fishes of South America. Porto Alegre; 2003.
Richard S, Arnoux A, Cerdan P, Reynouard C, Horeau V. Mercury levels of soils, sediments and fish in French Guiana, South America. Water Air Soil Pollut. 2000; 124:221–44. https://doi.org/10.1023/A:1005251016314
Roach KA. Environmental factors affecting incorporation of terrestrial material into large river food webs. Freshw Sci. 2013; 32(1):283–98. https://doi.org/10.1899/12-063.1
Roach KA, Jacobson NF, Fiorello CV, Stronza A, Winemiller KO. Gold mining and mercury bioaccumulation in a floodplain lake and main channel of the Tambopata River, Perú. J Environ Prot (Irvine, Calif). 2013; 4(1):51–60. https://doi.org/10.4236/jep.2013.41005
Roach KA, Winemiller KO, Davis SE. Autochthonous production in shallow littoral zones of five floodplain rivers: effects of flow, turbidity, and nutrients. Freshw Biol. 2014; 59(6):1278–93. https://doi.org/10.1111/fwb.12347
Roulet M, Guimarães JRD, Lucotte M. Methylmercury production and accumulation in sediments and soils of an Amazonian floodplain-effect of seasonal inundation. Water Air Soil Pollut. 2001; 128:41–60. https://doi.org/10.1023/A:1010379103335
Sanseverino A, Bastviken D, Sundh I, Pickova J, Enrich-Prast A. Methane carbon supports aquatic food webs to the fish level. PloS One. 2012; 7(8):e42723. https://doi.org/10.1371/journal.pone.0042723
Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO. 2007; 36(1):12–19. http://doi.org/10.1579/0044-7447(2007)36[12:eoemot]2.0.co;2
Singer MB, Harrison LR, Donovan PM, Blum JD, Marvin-DiPasquale M. Hydrologic indicators of hot spots and hot moments of mercury methylation potential along river corridors. Sci Total Environ. 2016; 568:697–711. https://doi.org/10.1016/j.scitotenv.2016.03.005
Singh D, Watson C, Mangal, S. Identification of the Sources and Assessment of the Levels of Mercury Contamination in the Mazaruni Basin in Guyana, in Order to Recommend Mitigation Measures. Institute of Applied Science and Technology. 2001; 1–10.
Souza LS, Armbruster JW, Willink PW. Connectivity of Neotropical river basins in the Central Guiana Shield based on fish distributions. Front For Glob Change. 2020; 3(8):1–15. https://doi.org/10.3389/ffgc.2020.00008
Sweet LI, Zelikoff JT. Toxicology and immunotoxicology of mercury: A comparative review in fish and humans. J Toxicol Environ Health B Crit Rev. 2001; 4(2):161–205. https://doi.org/10.1080/10937400117236
Telmer KH, Veiga MM. World emissions of mercury from artisanal and small-scale gold mining. In: Mason R, Pirrone N, editors. Mercury fate and transport in the global atmosphere. Boston: Springer; 2009. p.131–72.
Tsui MT, Finlay JC, Balogh SJ, Nollet YH. In situ production of methylmercury within a stream channel in northern California. Environ Sci Technol. 2010; 44(18):6998–7004. https://doi.org/10.1021/es101374y
UNEP. UNEP global mercury assessment 2013: Sources, emissions, releases and environmental transport [Internet]. Geneva; 2013. Available from: https://wedocs.unep.org/handle/20.500.11822/7984
van der Sleen P, Albert JS, editors. Field guide to the fishes of the Amazon, Orinoco, and Guianas. Princeton: Princeton University Press; 2018.
Veiga MM. Mercury in artisanal gold mining in Latin America: Facts, fantasies and solutions [Internet]. Vienna; 1997. Available from: https://content.sph.harvard.edu/mining/files/Veiga.pdf
Venturieri R, Oliveira-da-Costa M, Gama C, Jaster CB. Mercury contamination within protected areas in the Brazilian northern Amazon-Amapá State. Am J Environ Sci. 2017; 13(1):11–21. https://doi.org/10.3844/ajessp.2017.11.21
Walters D, Raikow DF, Hammerschmidt CR, Mehling MG, Kovach A, Oris JT. Methylmercury bioaccumulation in stream food webs declines with increasing primary production. Environ Sci Technol. 2015; 49(13):7762–69. https://doi.org/10.1021/acs.est.5b00911
Wantzen KM, Machado FA, Voss M, Boriss H, Junk WJ. Seasonal isotopic shifts in fish of the Pantanal wetland, Brazil. Aquat Sci. 2002; 64:239–51.
Watras CJ, Morrison KA, Kent A, Bloom NS. Chemical correlates of Hg and Methyl-Hg in northern Wisconsin lakewaters under ice-cover. Water Air Soil Pollut 1995; 84:253–67. https://doi.org/10.1021/es040561g
Watson LC, Hurtado-Gonzales JL, Chin CJ, Persaud J. Survey of methylmercury exposures and risk factors among indigenous communities in Guyana, South America. J Health Pollut. 2020; 10(26):200604. https://doi.org/10.5696/2156-9614-10.26.200604
Winemiller KO. Ecomorphological diversification in lowland of freshwater fish assemblages from five biotic regions. Ecol Monogr. 1991; 61(4):343–65. Available from: https://www.jstor.org/stable/2937046
Winemiller KO, Montaña CG, Roelke DL, Cotner JB, Montoya JV, Sanchez L, Castillo MM, Layman CA. Pulsing hydrology determines top-down control of basal resources in a tropical river-floodplain ecosystem. Ecol Monogr. 2014; 84(4):621–35. https://doi.org/10.1890/13-1822.1
World Health Organization (WHO). ‘‘Methylmercury’’ Environmental Health Criteria 101 [Internet]. Geneva; 1990. Available from: http://www.inchem.org/documents/ehc/ehc/ehc101.htm
Authors
Carmen G. Montaña1 ![]()
![]() , Elford Liverpool2
, Elford Liverpool2 ![]() , Donald C. Taphorn3
, Donald C. Taphorn3 ![]() and Christopher M. Schalk4
and Christopher M. Schalk4![]()
[1] Department of Biology, Stephen F. Austin State University, 2111 Raguet St. Nacogdoches, 75962, Texas, TX, USA. montanascg@sfasu.edu (corresponding author).
[2] Department of Biology, Faculty of Natural Sciences, University of Guyana, East Coast Demerara, 413741, Georgetown, Guyana. elford.liverpool@uog.edu.gy.
[3] 1822 N. Charles St., Belleville, 62221, Illinois, IL, USA. taphorn@gmail.com.
[4] Arthur Temple College of Forestry and Agriculture, Stephen F. Austin State University, 419 E College St, Nacogdoches, 75962, Texas, TX, USA. schalkc@sfasu.edu.
Authors Contribution 
Carmen G. Montaña: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing-original draft, Writing-review and editing.
Elford Liverpool: Conceptualization, Investigation, Methodology, Writing-review and editing.
Donald C. Taphorn: Investigation, Writing-review and editing.
Christopher M. Schalk: Conceptualization, Formal analysis, Investigation, Writing-review and editing.
Ethical Statement
The Staff of the Centre for the Study of Biological Diversity (CSBD) at the University of Guyana for the verification process of the fish and EPA-Guyana for processing the research and export permits. This study was conducted under EPA-Guyana permit No. 031516 BR003 to EL, CGM and DCT.
Competing Interests
The authors declare no competing interests.
How to cite this article
Montaña CG, Liverpool E, Taphorn DC, Schalk CM. The cost of gold: Mercury contamination of fishes in a Neotropical river food web. Neotrop Ichthyol. 2021; 19(3):e200155. https://doi.org/10.1590/1982-0224-2020-0155
Copyright
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Distributed under
Creative Commons CC-BY 4.0

© 2021 The Authors.
Diversity and Distributions Published by SBI
![]() Accepted May 26, 2021 by Fernando Carvalho
Accepted May 26, 2021 by Fernando Carvalho
![]() Submitted December 30, 2020
Submitted December 30, 2020
![]() Epub Sept 17, 2021
Epub Sept 17, 2021