![]() Jones Santander-Neto1
Jones Santander-Neto1 ![]() ,
, ![]() Nádia Ortolan da Vitória1,
Nádia Ortolan da Vitória1, ![]() Henrique David Lavander2 and
Henrique David Lavander2 and ![]() Andressa C. M. de Melo1
Andressa C. M. de Melo1
PDF: EN XML: EN | Supplementary: S1 S2 S3 | Cite this article
Associate Editor: ![]() Fernando Gibran
Fernando Gibran
Editor-in-chief: ![]() Carla Pavanelli
Carla Pavanelli
Abstract
O peroá, Balistes capriscus, é uma espécie associada a recifes que sustenta pescarias nas porções leste e oeste do Atlântico. No Brasil, esta espécie apresenta alta importância comercial e tem enfrentado reduções alarmantes de biomassa ao longo do tempo. Este estudo fornece uma atualização sobre os aspectos biológicos do peroá e sugestões de medidas de manejo no Brasil após 20 anos da primeira avaliação. Um total de 566 indivíduos foram obtidos em pescarias artesanais do sudeste do Brasil, desde o estado do Espírito Santo até o norte do Rio de Janeiro, de fevereiro de 2021 a fevereiro de 2023. Os peixes variaram de 153,4 a 419,0 mm de comprimento furcal (CF). A amostra foi composta principalmente por indivíduos maduros. As avaliações do índice gonadossomático sugerem que a época de desova ocorre principalmente entre dezembro e fevereiro, com pico em dezembro, coincidindo com o verão. O tamanho de maturidade é estimado em 230,9 mm CF (intervalo de confiança 225–234 mm). Com base nesses dados, sugerimos alterar o tamanho mínimo de captura para 230 mm CF (atualmente 200 mm CF) como uma medida de recuperação e gestão para pescarias de peroá no sudeste do Brasil e, se entendermos que os estoques atuais exigem mais medidas de recuperação, o estabelecimento de um período de defeso entre novembro e fevereiro.
Palavras-chave: Desenvolvimento ovocitário, Histologia, Oceano Atlântico, Peixe recifal, Pressão pesqueira.
Introduction
Tetraodontiformes are a morphologically and ecologically diverse order comprising over 430 fish species distributed throughout oceans, estuarine and freshwater zones (Tyler, 1980; Monteiro-Neto et al., 2003; Santini et al., 2013; Fricke et al., 2018). Most species are important coral reef and seagrass ecosystem components, playing diverse functional roles and indirectly influencing their habitat structures and compositions (Matsuura, 2014; Patankar et al., 2014; Darling et al., 2017; Stump et al., 2018; Eduardo et al., 2020). Tetraodontiformes fish have a long fisheries history, as they are an important source of food and income for small-scale fishing communities worldwide (Nóbrega et al., 2015; Stump et al., 2018). Fishing impacts, as well as diverse fish uses, habitat destruction and climate change, however, have severely impacted and decreased most Tetraodontiformes populations (Darling et al., 2017; Eduardo et al., 2020).
The gray triggerfish, Balistes capriscus Gmelin, 1789, is a Balistidae family member widely distributed worldwide, found on the west coast of the Atlantic from Canada to Argentina and on the east coast from Ireland to Angola, including the Mediterranean Sea. This species inhabits hard bottoms, reefs, and ledges and has also been found on artificial reefs, at various depths up to about 110 m deep, but generally ranging between 15 and 55 m (Liu et al., 2015).
Gray triggerfish fisheries are of considerable economic and social importance in eastern and western portions of the Atlantic Ocean, including the Mediterranean Sea, Gulf of Mexico and Caribbean Sea (Moore, 2001; SEDAR, 2006; Aggrey-Fynn, 2009, 2013; Kacem, Neifar, 2014; Burton et al., 2015; Kacem et al., 2015; Kelly-Stormer et al., 2017), including Brazil (Bernardes, Dias, 2000; Bernardes, 2002; Castro et al., 2005; Vianna et al., 2007; Hostim-Silva, Soares, 2013). According to the latest International Union for Conservation of Nature (IUCN) assessment, the gray triggerfish, Balistes capriscus, is globally categorized as Vulnerable (VU), mainly due to fisheries activities, leading to stock overexploitation. Although classified as Near Threatened (NT) in Brazil (ICMBio, 2024), reduced gray triggerfish landings have been observed from 1986 to 2005, reaching 95% decreases in the states of Santa Catarina and Rio Grande do Sul (Ataliba et al., 2009). The species is also classified as Vulnerable (VU) by the Espírito Santo State list of endangered fauna and flora (Fraga et al., 2019; Hostim-Silva et al., 2019). Throughout the Brazilian coast, it is most abundant from southern Bahia to Rio Grande do Sul (Castro et al., 2005). Although it presents high commercial importance and has faced alarming biomass decreases over time, only a few studies are available to date concerning gray triggerfish fisheries and ecology, such as growth, reproduction, and habitat use (Kelly-Stormer et al., 2017; Eduardo et al., 2020), are available to date.
Studies on the reproductive biology of commercially important aquatic species are essential to understand reproductive aspects, including habitat use dynamics, and establish or change management measures. For example, minimum catch sizes and closed seasons, aiming at their recovery and supporting stock assessments with model input data to define a given species threat status and sustainable use potential (Sparre, Venema, 1997; Fonteles-Filho, 2011; King, 2013). These types of assessments should be conducted at regular intervals and in different areas to understand how density-dependence mechanisms act on populations and alter their biological parameters (Hilborn, Walters, 1992; King, 2013).
In Brazil, guidelines on gray triggerfish uses are based on population biology studies dating back to the mid-1980s (Bernardes, Dias, 2000; Bernardes, 2002). Considering that species must be evaluated within a certain generational interval (IUCN, 2024), and that these biological data are beyond the recommended 24-year interval (Liu et al., 2015), this study aimed to investigate gray triggerfish reproductive aspects in southeastern Brazil and contribute to fisheries management by updating current management measures, as well as suggesting new measures.
Material and methods
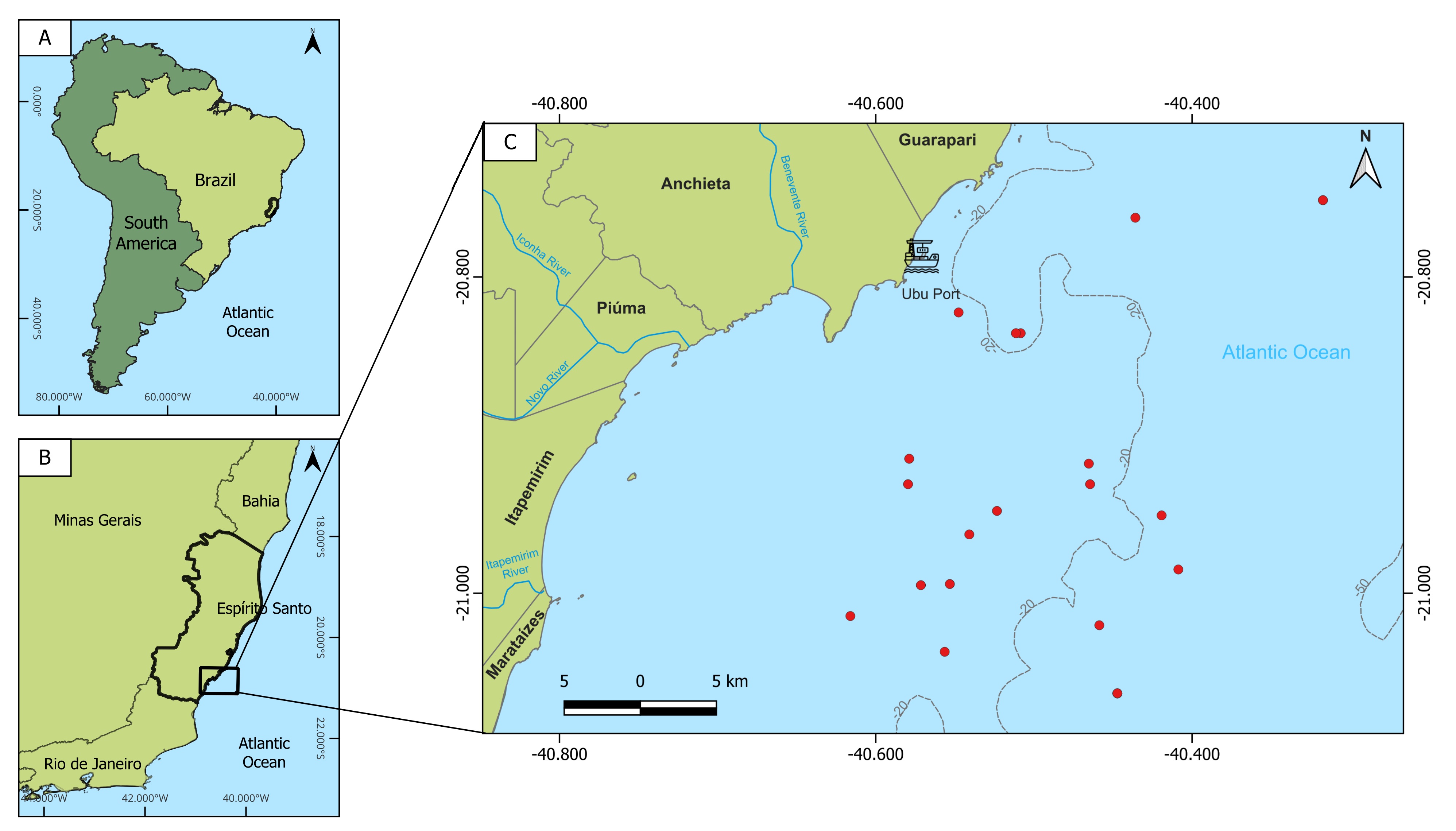
A total of 566 specimens were analyzed between February 2021 and February 2023, obtained through monitoring landings from commercial fisheries in the state of Espírito Santo, in the municipalities of Conceição da Barra, Vitória, Guarapari, Piúma and Marataízes, and scientific shipments in the municipality of Piúma (Fig. 1). Gray triggerfish, Balistes capriscus, specimens were captured by a specific type of bottom line, locally known as the pargueira, composed of 10 to 15 pairs of 25 hooks per line. The smallest individuals (> 20 cm in fork length) were captured with hand nets (puçás). Animal welfare laws, guidelines or policies were not applicable because the sample collected during the present study was based on landed specimens being sold to the public by fishermen. Five specimens were deposited at the Laboratório de Dinâmica de Populações Marinhas at the Instituto Federal de Ciência e Tecnologia do Espírito Santo (Piúma campus), Brazil, under the catalog number: BCA 001. At the laboratory, fork length (FL, cm), total weight (TW, g), liver weight (LW, g), and gonad weight (GW, g) were recorded. The expected 1:1 sex ratio was tested by the Chi-square χ² test (p < 0.05) and differences between sexes concerning fork length (FL) and TW were determined by Student’s t-test (p < 0.05). The weight and length ratio were used to establish and compare relationships between FL and TW for each sex (α = 0.05).
FIGURE 1| Brazilian coast highlighting the Espírito Santo State (A) and its South region (B) with the sampling locations (C) of the gray triggerfish Balistes capriscus (red points).
Sexes were determined by microscopic gonadal evaluations. Maturation stages were categorized according to a marine fish classification protocol adapted for the species (Brown-Peterson et al., 2011). Following this analysis, gray triggerfish gonads (ovaries and testes) were fixed in a 10% formaldehyde solution for up to 48 h and transferred to a 70% alcohol solution. The samples were then routinely dehydrated in an increasing alcohol gradient (80%, 90%, 95%, and 100%) (Junqueira, Carneiro, 2013), diaphanized in xylene, and finally embedded in paraffin at 57ºC. The blocks were cut transversally (5 μm thickness) employing a rotary microtome (LUPETEC MRPSA2016) and stained with Hematoxylin and Eosin (Junqueira, Carneiro, 2013). Different maturity stages were determined through germline components using photomicrographs obtained with an HD camera and analyzed using the SIGMA Scan Pro 5.0 software.
The logistic curve that describes the relationship between the amount of mature individuals in each furcal length interval was obtained through the applied microscopic development stage classifications. The size of the first gonadal maturation (L50%) and maximum maturity length (L99%) were estimated according to the following model: P = 1/(1 + e-(a+b*FL)), with the R software (v. 4.3.3) (R Development Core Team, 2024) and the FSA package (Ogle et al., 2023). An Analysis of Covariance (ANCOVA) test was used to compare the L50% estimate curves between sexes.
Gray triggerfish reproductive periods were determined by analyzing the relative frequency of each maturation stage and monthly Gonadosomatic Index (GSI) variations, determined as GSI (%) = (GW/TW) x 100. The index and the monthly average values were calculated for each individual. Monthly GSI variations indicate GW evolution throughout the year and allow for the identification of different sexual cycle stages. Additionally, monthly liver weight variations were assessed by the Hepatosomatic Index (HSI), calculated as HSI = (LW/TW) x 100. An analysis of variance (ANOVA) test was used to determine potential significant differences between months of the year. Tukey’s post-hoc test was used to determine potential differences between groups. The monthly number of female development stages was calculated to determine peak spawning months.
Results
The sex ratio of the total sample (n = 566) of Balistes capriscus did not differ statistically from the expected, totaling 293 females and 258 males (1.14♀:1♂; X² = 2.223, p-value = 0.1475). The sex of 15 specimens could not be determined. No sex ratio variations were observed throughout the year, except for June (X² = 5, p-value = 0.0369) and September (X² = 14.4, p-value = 0.0003), in which the sample was mostly composed of females. No sex ratio differences according to length classes were observed, except for the classes between 260 and 280 mm FL (1.94♀:1♂; X² = 9.907, p-value = 0.0023), in favor of females, and 320 and 340 mm FL (0.2♀:1♂; X² = 5.333, p-value = 0.0433), in favor of males.
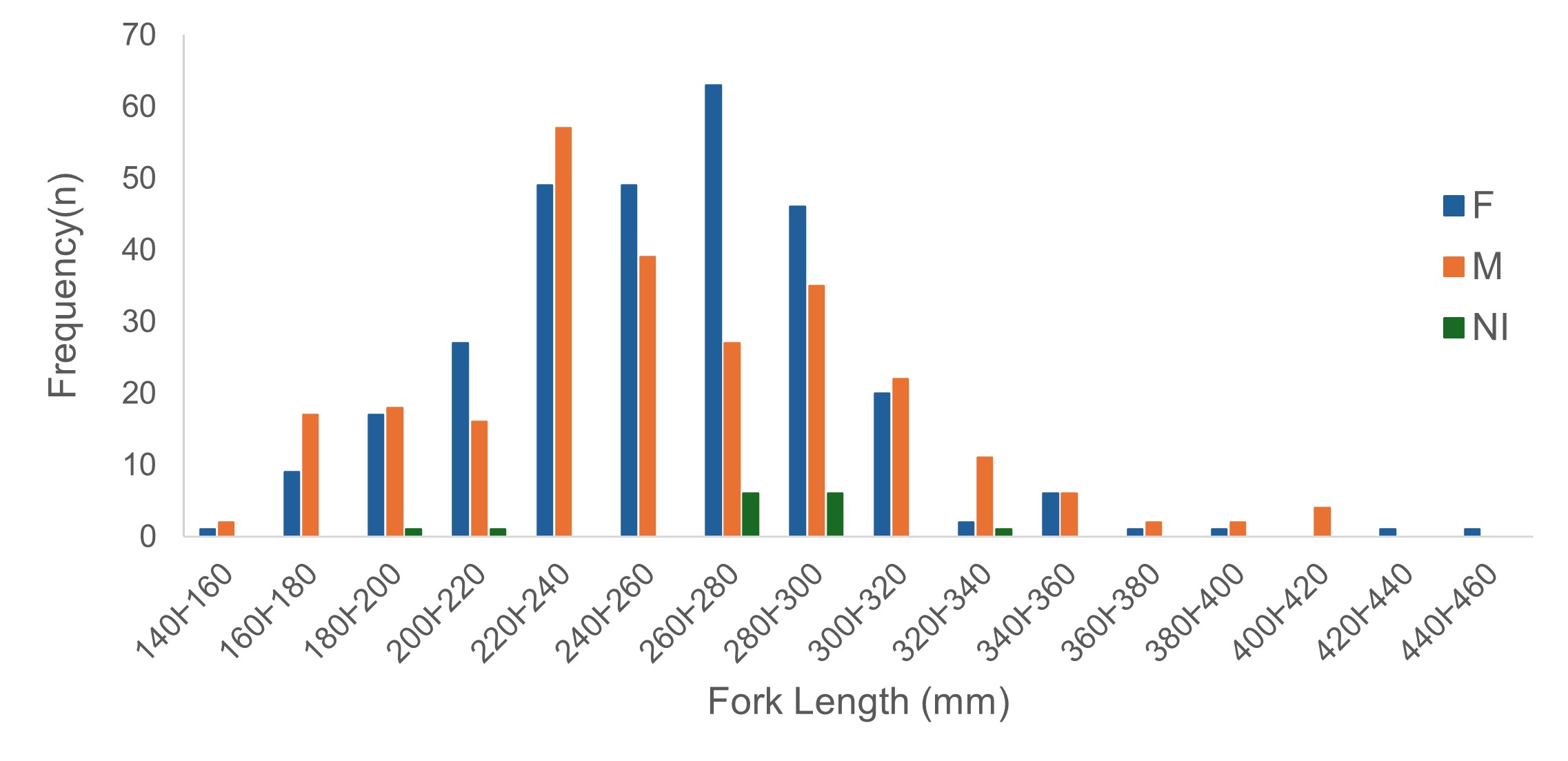
Male lengths ranged from 153.4 to 419.0 mm FL (mean = 256.12 ± 49.83 mm) with a predominance of lengths between 220 and 300 mm FL and modal class of 220 to 240 mm FL, while female lengths ranged from 154.8 to 440 mm FL (mean = 255.54 ± 45.47 mm), with a predominance of lengths between 220 and 300 mm FL and modal class of 260 to 280 mm FL (Fig. 2). No significant differences between mean FL values for males and females were observed (Student’s t = 0.1340, p-value = 0.8936). No differences between median weights were noted (males: 305 g, females: 330 g; Mann-Whitney, Z = 0.1589; p-value: 0.8737). Gray triggerfish presented negative allometric growth for both sexes (b < 3) (Fig. S1), with no difference between FL x TW correlations between males and females (ANCOVA, p-value = 0.44), with a confidence interval of 2.80–2.90 for both sexes. The equation for male was TW = 4.97×10-5FL2.836 (n = 228; R2 = 0.93), for female TW = 3.933×10-5FL2.881 (n = 285; R2 = 0.96), and for both sexes TW = 4.528×10-5FL2.855 (n = 523; R2 = 0.95).
FIGURE 2| Length structure of the gray triggerfish, Balistes capriscus, captured in southeast Brazil. Blue bars: F, females (n = 293); orange bars: M, males (n = 258); green bars: NI, not identified sex (n = 15).
A total of 277 gonads (females, n = 162; males, n = 85) were microscopically evaluated, with sex and reproductive phase assigned to each individual, when possible. Five gray triggerfish gonadal development stages were described macroscopically and microscopically, as I – immature; II – developing; III – spawning capacity; IV – regressing and V – regenerating (Tab. 1). Individuals in stages I and II were considered immature, while individuals between stages III and V were considered mature.
TABLE 1 | Microscopic evaluation used to classify the reproductive phase of the gray triggerfish, Balistes capriscus, adapted from the classification of Brown-Peterson et al. (2011) and Kelly-Stormer et al. (2017).
Reproductive stages | Female | Male |
I – Immature | Oogonia and pre-vitellogenic oocytes, thin ovarian wall and ovarian parenchyma at the initial level of organization of the ovigerous lamellae | Primary spermatogonia visible in the cortical portion of the organ; little or no development of spermatocytes, reduced lobules and ducts |
II – Developing | Pre-vitellogenic oocytes in abundance, oocytes in initial and secondary vitellogenesis, thin ovigerous lamella occupying the ovarian parenchyma, ovarian wall starting to vary from thick to thin | Spermatogonia and spermatocytes visible; elongation of the lobules with some development of sperm in the testes |
III – Spawning capable | Abundant mature oocytes with abundant vitellogenic vesicles, higher frequency of oocytes in advanced stages of development, presence of hydrated oocytes and post-ovulatory oocytes; disorganization of the ovigerous lamella and thin ovarian wall | Spermatozoa evident in the testes and spermatic ducts; ducts developed and expanded |
IV – Regressing | Vitellogenic oocytes mostly in the process of atresia disorganization of the ovigerous lamellae, thin ovarian wall with a loose appearance | Reduced spermatogenesis and residual sperm in ducts and lobules |
V – Regenerating | Oogenesis restarts with abundant pre-vitellogenic oocytes and traces of atresia, ovigerous lamellae in the process of reorganization invading the ovarian parenchyma, thick ovarian wall | Spermatogenesis with abundant initial lineages (spermatogonia and primary spermatocytes), little or no development of spermatocytes; empty duct and lobules |
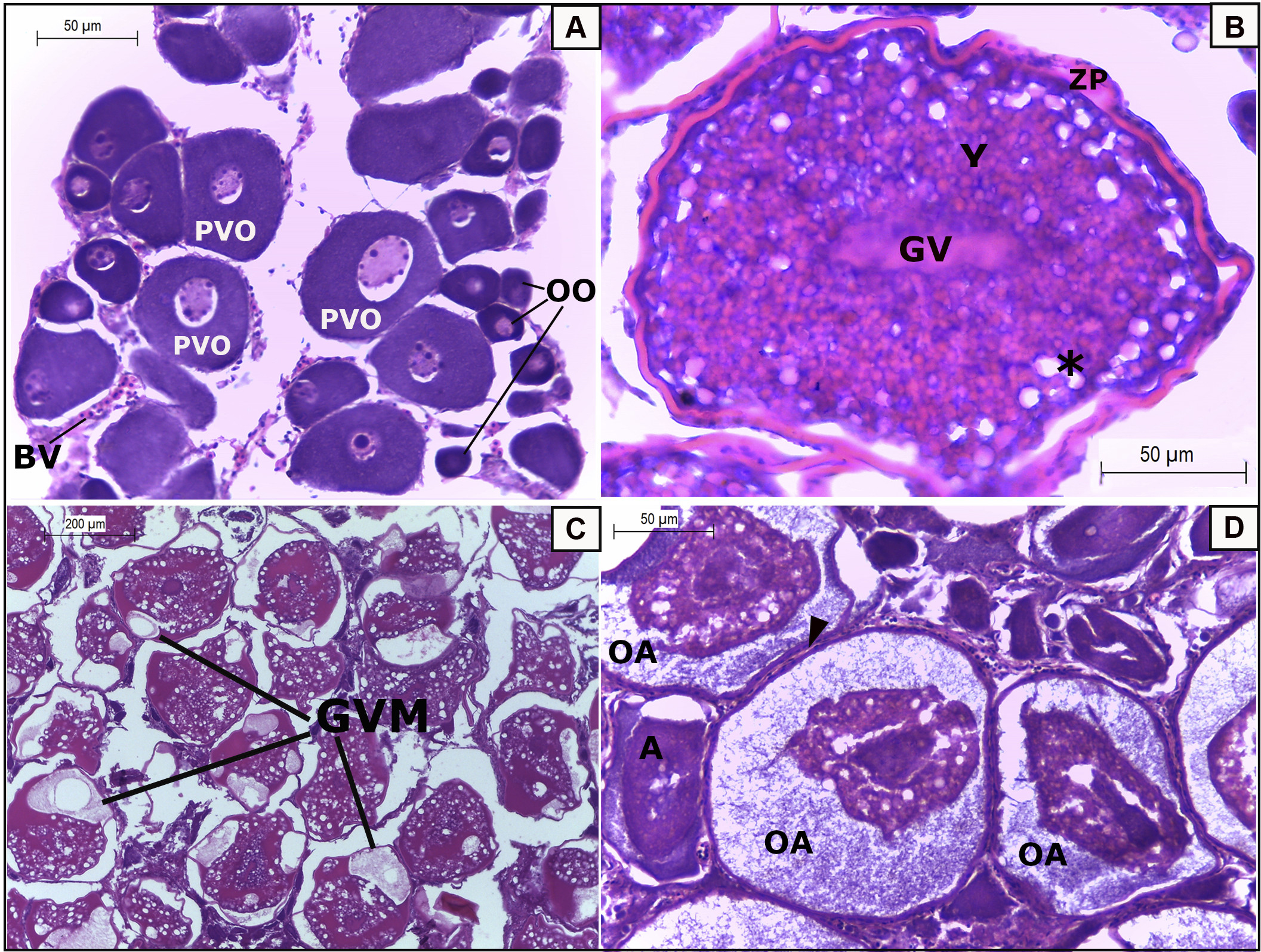
Microscopically, the ovaries and testes are similar to those described for this species at different development stages (see Kelly-Stormer et al., 2017). Germ cells were classified as oogonia (OO) (Fig. 3A), pre-vitellogenic oocytes (PVO) (Fig. 3A), vitellogenic oocytes (V) (Fig. 3B), oocytes with germinal vesicle migration (GVM) (Fig. 3C), oocytes in absorption (Fig. 3D) and oocytes in atresia (A) (Fig. 3D). The ovaries of immature females showed a predominance of oogonia (OO) and pre-vitellogenic oocytes (PVO) (Fig. 4A), a germinal zone occupying the cortical ovary zone, beginning the organization of ovigerous lamellae (OL) and thin ovarian walls (OW) (Fig. 4A). Early developing ovaries present a predominance of previtellogenic oocytes (PVO) (Fig. 4B), and as they approach final development, vitellogenesis (V) oocytes are mostly found in the ovigerous lamellae, which expand to the medullary portion of the oocyte as the oocytes develop, delimited by a thick ovarian wall (OW) with an abundance of blood vessels (BV) (Fig. 4C). Spawning-capable ovaries contain mostly oocytes presenting germinal zone migration (GVM) with abundant cytoplasm vitellogenic vesicles, in addition to post-ovulatory follicles (Fig. 4D, arrow) characterized by basement membrane degeneration, suggesting imminent spawning. The ovarian wall (OW) is thin and loose (Fig. 4D). Despite the presence of GVM and post-ovulatory follicles, no hydrated oocytes were observed in spawning-capable females.
FIGURE 3| Oocyte development from germ line cells of gray triggerfish, Balistes capriscus captured in southern Espírito Santo, Brazil. A. Oogonia (OO), previtellogenic oocytes (PVO) and blood vessels (BV) observed in immature females. B. Vitellogenic oocyte highlighting the germinal vesicle (GV), abundance of yolk (Y), zona pellucida (ZP), and vacuole lipids (*) characteristic of females in final development stage. C. Oocyte with migration of the germinal vesicle (GVM) characteristic of spawning capable females. D. Oocyte in absorption (OA), oocytes in atresia (A) observed in spawning capable females and in regression. Arrowhead indicates the follicle cells.
FIGURE 4| Photomicrograph of the different stages of ovarian development of the gray trigger fish Balistes capriscus, captured in southeast Brazil. A. Immature: thin ovarian wall (OW), with the presence of blood vessels (BV), oogonia (circle) and pré-vitellogenic oocytes (PVO) delimited by an ovarian lamella (OL). B. In early development: ovarian wall (OW) in the process of thickening, pre-vitellogenic oocytes (PVO) in abundance; C. in final development: completely thickened ovarian wall (OW), with the presence of enlarged blood vessels (BV), vitellogenic oocytes (V) occupying the entire ovarian parenchyma, in addition to the presence of the first postovulatory oocytes (arrowhead). D. Spawning capable: loose and less thick ovarian wall (OW), presence of vitellogenic oocytes (V), oocytes with migration of the germinal vesicle (GVM) and post-ovulatory oocytes (arrow). E. Regression: loose ovarian wall (OW) delimiting an ovarian parenchyma with oocytes in atresia (arrowhead), in addition to the other cells of the germline. F. Regeneration: thick ovarian wall (OW), visible ovigerous lamella (OL) with predominance of pre-vitellogenic oocytes (PVO), visible interlamelar space (IS), abundant blood vessels (BV), in addition to the presence of oogonia (circle).
Regressing ovaries contain remaining vitellogenic oocytes undergoing the atresia process (Fig. 4E), in addition to the presence of pre-vitellogenic oocytes (PVO). At this stage, the ovarian wall (OW) presents the same features as in the previous stage (spawning-capable), with disorganized ovigerous lamella and a loose ovarian wall with bundles invading the ovarian parenchyma (Fig. 4E). Regenerating ovaries present features and oocyte compositions similar to those of development ovaries, except for the ovarian wall (OW), which is more vascularized, the presence of abundant blood vessels (BV) in the ovarian parenchyma, and visible interlamelar space (IS) (Fig. 4F).
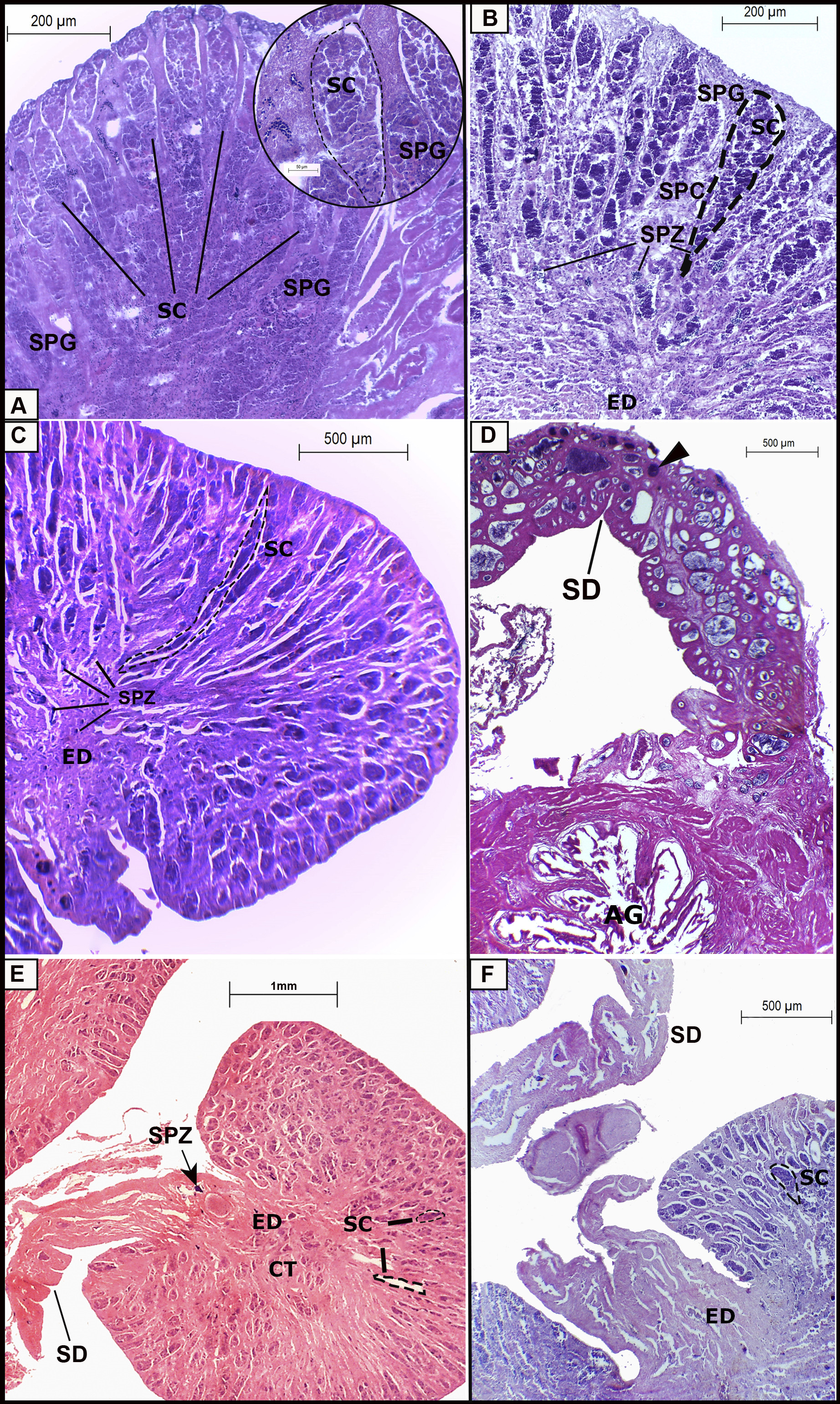
The microscopic appearance of immature testes is compact, with little differentiation of spermatic lobules or cysts. This organ is composed predominantly of spermatogonia (SPG) distributed mostly in the cortical and medullary testes portions (Fig. 5A). Developing testes present spermatic cyst (SC) elongation and differentiation. Abundant spermatogonia are visible in the cortical portion of the testis, while developing spermatocytes (SPC) and some spermatozoa (SPZ) are distributed up to the medullary portion of this organ (Fig. 5B). The efferent duct (ED) is differentiated, but contains few spermatozoa (Fig. 5B). In spawning-capable testes, spermatozoa (SPZ) are visible in spermatic cysts (Fig. 5C) and in the spermatic duct (SD) (Fig. 5D). Spermatogenesis reaches its maximum production with all lineage cells observed in the organ. In the cortical portion of the testes, spermatic cysts (SC) reach their maximum elongation up to the medullary portion (Fig. 5C). A higher frequency of spermatogonia and primary spermatocytes is observed in the first portion while more spermatozoa (SPZ) are observed towards the medullary portion, filling the spermatic cysts (SC) (Fig. 5C). The spermatic duct (SD) becomes more visible, progressively expanding, densely filled with packaged spermatozoa (Fig. 5D). The accessory gland (AG) presents a visible and active secretory epithelium (Fig. 5D). In regressing testes, cysts (SC) and the efferent duct (ED) contain remaining spermatozoa (SPZ) or are empty (Fig. 5E), with limited spermatogenesis and a proliferation of connective tissue (CT) in the medullary portion (Fig. 5E). A new spermatogenic lineage begins in regenerating testes, similar to that observed for developing testes, differing in lobule extension (Fig. 5F) and in efferent duct (ED) elongation.
FIGURE 5| Different stages of development of the male reproductive system of the gray triggerfish, Balistes capriscus, in southeastern Brazil. A. Immature: little differentiation of spermatic cysts (SC), with predominance of differentiating spermatogonia (SPG) (detail in the image). B. In development: enlargement of the spermatic cyst (SC), differentiation of spermatogonia (SPG) into spermatocytes (SPC) and presence of few spermatozoa (SPZ) erecting toward the medullary portion of the testis. C. Spawning capable: elongated spermatic cysts (SC) reaching the medullary portion of the testis, visible spermatozoa (SPZ) filling the efferent duct (ED). D. The spermatic duct (SD) is developed with the presence of packaged spermatozoa (arrowhead) and the accessory gland (AG) with apparent secretory activity. E. Regression: limited spermatogenesis with regression of spermatic cysts (SC) and proliferation of connective tissue in the testis, the presence of remaining spermatozoa (SPZ) and spermatic duct (SD) with an emptied appearance is observed. F. Regeneration: new spermatogenic lineage begins with enlargement of spermatic cysts (SC), efferent duct (ED) and spermatic duct (SD) with an emptied appearance.
A total of 162 females (FL range 154.7 to 440.0 mm) and 85 males (FL 166.8 to 419.0 mm) were used to estimate gray triggerfish maturity sizes. Lengths at first maturity (L50%) did not differ between sexes (ANCOVA, z = 0.49, p-value = 0.22), estimated as 230.9 mm FL (confidence intervals: 225-234 mm). Although no difference between sexes was noted, the L50% of males was slightly higher (231.6 mm FL) than that of females (227.3 mm FL) (Fig. S2). The smallest mature female measured 209 mm FL, while the smallest mature male measured 210 mm FL, and the largest immature female, 252 mm FL, while the largest immature male measured 257 mm FL. A total of 71% of the total sample was composed of mature individuals, 70% of which were mature females, while a total of 71% of males were mature.
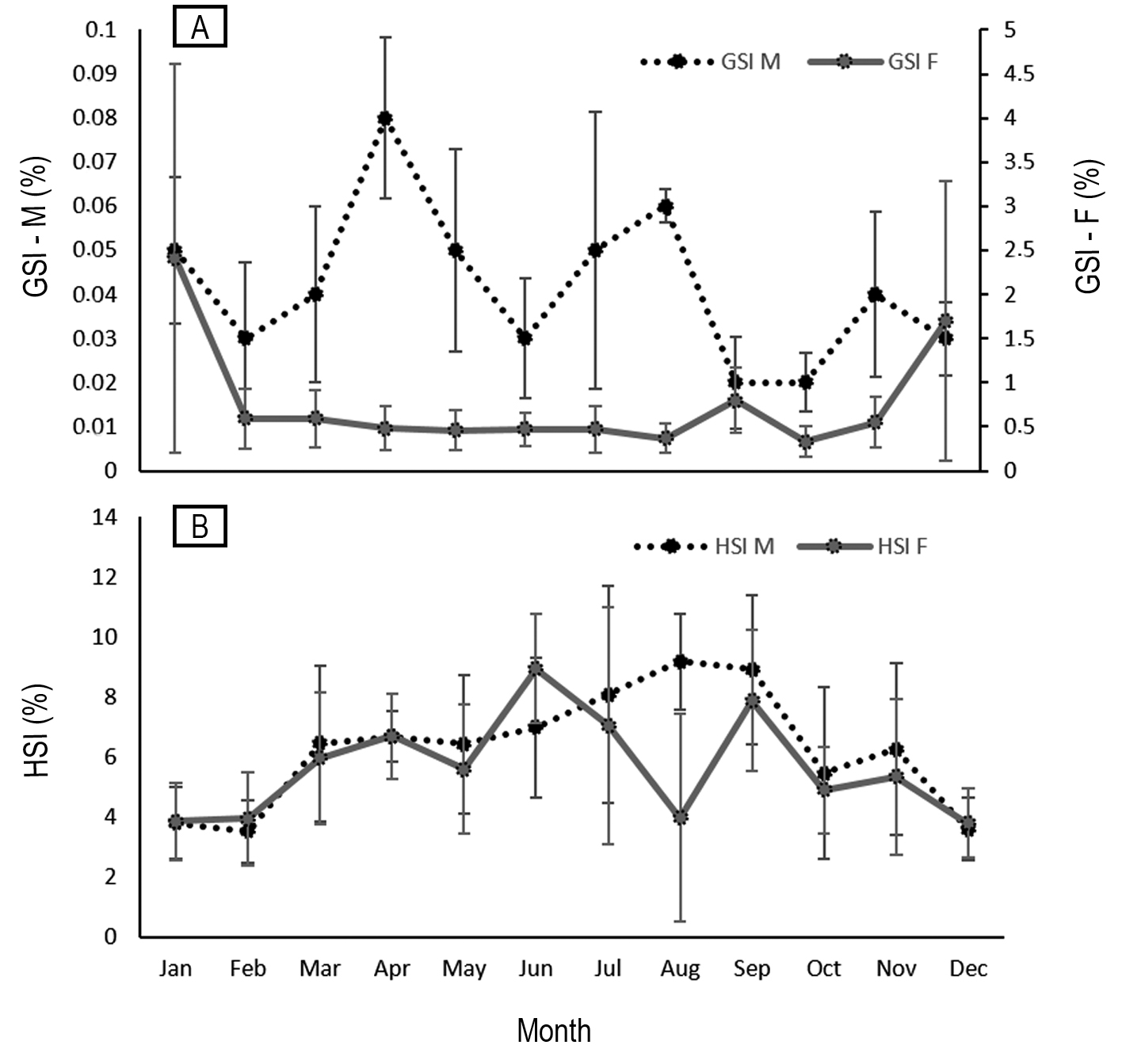
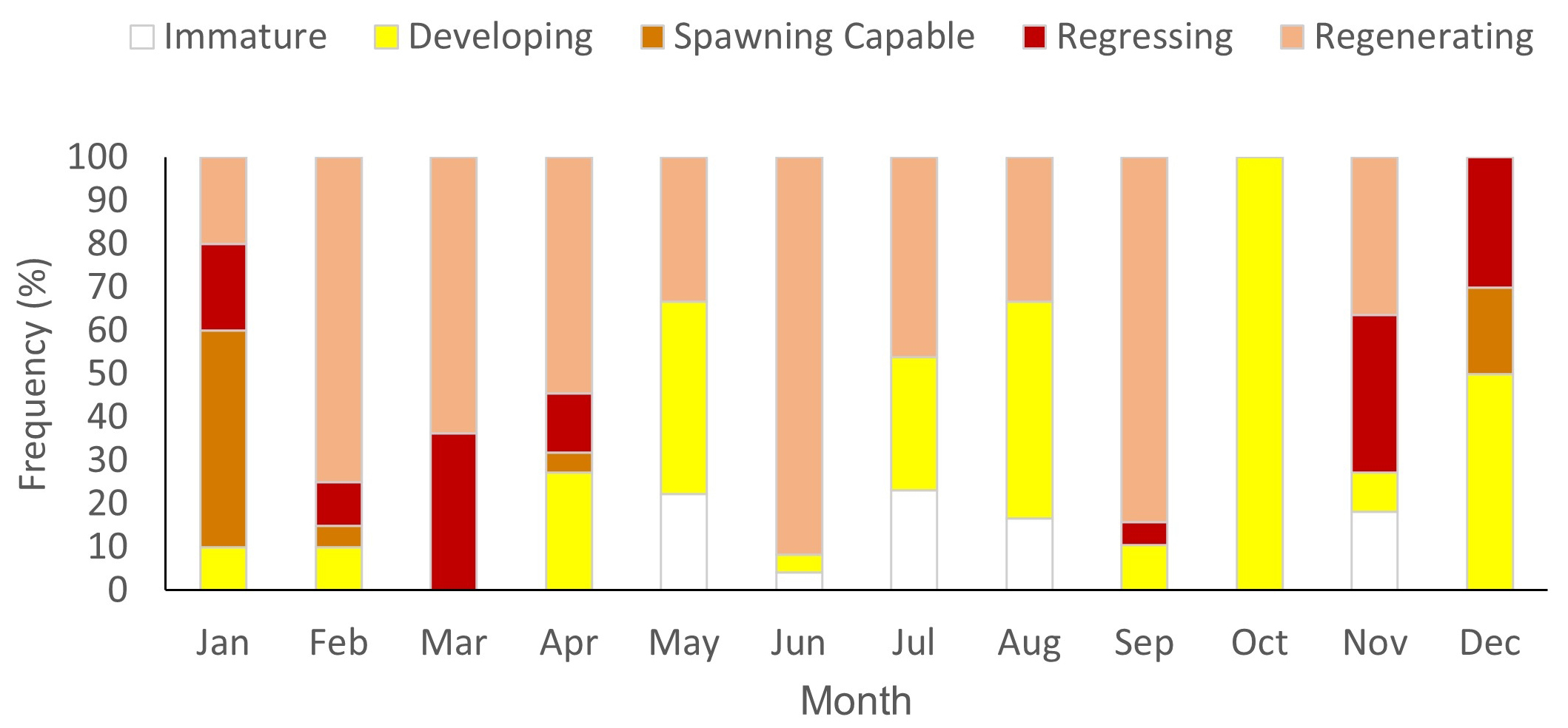
The determined GSI values ranged from 0.04 to 6.73 for females and from 0.01 to 0.11 for males. The highest GSI values for females were observed between November and January, peaking between December and January (Fig. 6A). Declining GSI values were noted from February onwards, increasing again between September and October. In males, the highest GSI values were recorded in April, August and November (Fig. 6A). The HSI ranged between 0.98 and 12.35 for females and 1.23 to 13.42 for males. A gradual increase in HSI values was observed between January and April for both sexes, decreasing in May, and peaking in June for females and in August for males (Fig. 6B). The minimum values of this index were observed in August for females and between November and December for both sexes. A gradual HSI increase was observed during months in which GSI values did not vary significantly for females (Fig. 6B), gradually declining during a higher GSI peak, between December and January, when HSI reductions were also observed, potentially associated with gray triggerfish reproductive activity, when energy demands are high. In the study area, regenerating females (n = 98) represented about 53% of the total sample, and were found during all months of the year, except for October and December, with a greater frequency noted from February to June and in September (Fig. 7). Spawning-capable females represented about 5% (n = 9) of the sample and were observed from December to February and in April, when the highest GSI values were recorded, alongside females presenting oocytes in absorption (December), indicative of recent spawning. The presence of regressing females (n = 19) between September and April (Fig. 7) and decreased GSI values observed from February onwards indicate that the spawning period for this species probably begins in December and continues until February.
FIGURE 6| Monthly variations in the gonadosomatic (GSI) (A) and hepatosomatic (HSI) (B) indices, mean values (± standard deviation) for both sexes of the gray triggerfish Balistes capriscus in Espírito Santo and northern Rio de Janeiro. GSI – F, females; GSI – M, males; HSI – F, females; HSI – M, males.
FIGURE 7| Percentage of females by gonadal development stage of Balistes capriscus monitored from April 2021 to January 2023 in Espírito Santo and northern Rio de Janeiro, Brazil.
Discussion
This study provides important data on reproductive aspects of the gray triggerfish, Balistes capriscus, population exploited in part of southeastern Brazil. Considering that the previous biological information for this species was estimated from samples collected in the mid-1980s and that dense dependence mechanisms may have occurred, the data reported herein are important for stock assessments and essential to fisheries management of the gray triggerfish in the South Atlantic.
The sex ratio in the study region did not differ from the expected 1:1 ratio, as noted in Southeastern USA for handline fishing samples (Moore, 2001), in the Gulf of Mexico for spearfishing samples (Lee, 2019) and in Iskenderun Bay, Turkey, for trawl samples (Işmen et al., 2004). A predominance of females was observed in Southeastern USA using chevron traps through fishery-dependent (Kelly-Stormer et al., 2017) and fishery-independent (Moore, 2001) sampling efforts, in the Gulf of Mexico using mainly traps and hook-and-line fisheries (Fitzhugh et al., 2015) and in the Gulf of Gabès in Tunisia using pelagic trawl nets (Kacem, Neifar, 2014). Some older studies, however, reported the predominance of males in the Gulf of Mexico (Wilson et al., 1995; Hood, Johnson, 1997), similar to the study carried out in the mid-1980s in São Paulo, Brazil using pair fishing (Bernardes, 2002). Considering only the total sample analyzed herein, it would be unwise to attempt to define a sex ratio pattern for gray triggerfish. However, some aspects are noteworthy. For example, some studies may or may not have indicated differences due to a relatively low number of samples (Wilson et al., 1995; Işmen et al., 2004; Lee, 2019). Furthermore, it is difficult to obtain information on the exact collection point of the specimens to assess sexual segregation according to habitats in commercial fisheries, as is the case for most studies, except for the Gulf of Mexico in experimental fisheries, where alternating sexual segregation was observed depending on proximity to the coast (Lee, 2019). However, it is interesting to note that the predominance of males is reported in studies carried out more than 25 years ago, and that a certain predominance, when present, was of females in more recent studies.
Most studies on gray triggerfish have not assessed sex ratio differences throughout the year. In the present study, ratio differences were observed in favor of females in June and September, corresponding to the end of autumn and winter in the southern hemisphere. This is the opposite of what was reported in Tunisia (Kacem, Neifar, 2014), where sex ratio differences were not observed only in the winter months. Without more detailed analyses of several environmental and biological parameters, causes for these differences cannot be postulated, especially when the only applied environmental indicator (season of the year) presented contrasting results.
The smallest individuals collected in the present study using a pargueira (a type of handline with several hooks) were much larger than the smallest specimens reported in southeastern USA (82 mm FL) caught using chevron traps (Kelly-Stormer et al., 2017) and in Turkey (79 mm FL) caught using trawls (Işmen et al., 2004). This is due to the different selectivity of these fishing devices, with the pargueira selecting larger individuals. It is important to note that no information on the occurrence locations of juvenile gray triggerfish in the study area is available to date. We believe it is also possible that juveniles do not occur in the area and migrate to these fishing locations. In Brazil, the ranges of lengths and minimum and maximum sizes reported herein were similar to those observed for purse seines and trawl nets in the states of Rio de Janeiro and São Paulo (Bernardes, 2002; Castro et al., 2005), and greater in length than fish captured with portable liftnets in the states of Rio de Janeiro and Espírito Santo. The fishery using portable liftnets, however, displays the potential to increase fishing efforts to the point of causing stock decreases over time, due to the capture of entire fish schools without excluding juveniles (Vianna et al., 2007). As it is a non-selective fishing technique that captures many small individuals, it was prohibited for gray triggerfish fisheries in Southeastern and Southern Brazil (IBAMA, 2002).
Gray triggerfish in the study area present a negative allometric growth, as observed in most studies carried out in southeastern USA, Ghana, Africa and Iskenderun Bay, in Turkey (Işmen et al., 2004; Aggrey-Finn, 2007; Burton et al., 2015). In those studies, the allometry coefficient was calculated as around 2.80–2.90, except for Ghana, calculated as 2.5. In Southeastern Brazil, however, isometric growth with a 3.08 coefficient was observed (Castro et al., 2005). These differences across different areas and periods can be due to nutritional fish conditions over time and space (Işmen et al., 2004).
The histological evaluations of the gonadal stages carried out herein aided in identifying fish sex, especially males presenting in initial development stages few macroscopic changes. Few assessments on the reproductive biology of this species are, in fact, based on microscopic gonadal development criteria (Bernardes, Dias, 2000; Lang, Fitzhugh, 2015; Kelly-Stormer et al., 2017). This may imply in the use of less accurate and subjective techniques (Brown-Peterson et al., 2011), which, if associated with other factors, may result in reproductive period estimate variations for this species in different regions (Kelly-Stormer et al., 2017). The gray triggerfish is a gonochoric species and females exhibit synchronous oocyte development by group (Ingram 2001; Lang, Fitzhugh, 2015; Kelly-Stormer et al., 2017). Regarding the latter, as observed in other studies (Lang, Fitzhugh, 2015; Kelly-Stormer et al., 2017), the presence of groups of oocytes undergoing secondary development suggests an established pattern for synchrony occurrence by group (see Wallace, Selman, 1981) in the study area. These two oocyte groups are clearly distinguishable (Fig. 3), with larger groups of oocytes in advanced development in synchrony, while the smaller group is more heterogeneous, presenting smaller oocytes (Figs. 3B, D–E). Although this pattern indicates a certain type of fecundity for most fish species (Brown-Peterson, 2003; McBride et al., 2015) there is no evidence of this for gray triggerfish, categorized until now as presenting indeterminate fecundity (Lang, Fitzhugh, 2015). Based on our findings, we suggest that detailed analyses of vitellogenic oocyte diameter variations during the spawning period be performed to confirm this asynchronous oocyte development pattern in the study area.
The occurrence of females with hydrated oocytes, which are used to identify batch spawning, is reported as rare in gray triggerfish studies (Hood, Johnson, 1997; Bernardes, Dias, 2000; Moore, 2001; Lang, Fitzhugh, 2015) and was not observed by us. Some factors may be associated with this low frequency, namely (i) the absence of hydrated oocytes during proteolysis (Lang, Fitzhugh, 2015), (ii) the short time between final oocyte maturation, hydration and ovulation (less than 24 h) (Brown-Peterson et al., 2003) making it difficult to capture females at the precise moment of this phase and (iii) possible spawner displacement to deep areas less accessible to fishing or reduced feeding during spawning, resulting in low susceptibility of females being capture with bait (Wilson et al., 1995; Bernardes, Dias, 2000; Simmons, Szedlmayer, 2012). In this sense, the presence of oocytes undergoing absorption, oocytes presenting germinal zone migration (MVT), and post-ovulatory follicles observed herein were used as indicative of the spawning period for gray triggerfish in the study area. The reproductive strategies observed for balistids, such as group oocyte development, indeterminate fecundity and batch spawning, are shared by several triggerfish species, such as the gray triggerfish (Lang, Fitzhugh, 2015; Kelly-Stormer et al., 2017; present study), queen triggerfish, Balistes vetula Linnaeus, 1758 (Hernández, Shervette, 2024), black triggerfish Melichthys niger (Bloch, 1786) (Branco et al., 2013) and red-toothed triggerfish Odonus niger (Rüppell, 1836). These strategies, combined with nesting and protection behavior, may allow for high reproductive output (Hernández, Shervette, 2024), thus ensuring triggerfish population maintenance.
The male reproductive system of the gray triggerfish presents unique characteristics associated with sperm storage, consisting of the testes, sperm duct, efferent duct and accessory gland. In spawning-capable males, unlike what is noted in other teleosts, the sperm produced in the testes do not accumulate in the sperm cysts or sperm duct (White et al., 1998; Brown-Peterson et al., 2011; Shinozaki-Mendes et al., 2013a), but rather in the accessory gland, where they are stored until copulation (Moore, 2001). In this study, the sperm duct did not vary in size between developing and mature individuals, but the efferent duct that transports the sperm to the accessory gland widens in mature individuals as it is filled with these cells. The sperm storage pattern of the gray triggerfish is associated with the harem behavior of the species, in which a male builds several nests in different locations and the accessory gland is necessary for sperm storage for prolonged periods to ensure successful egg fertilization in each nest (Simmons, Szedlmayer, 2012; Kelly-Stormer et al., 2017). Because of this, testes and accessory organ assessments are paramount to understand the reproductive cycle of gray triggerfish males.
Reported estimated maturity sizes for gray triggerfish, Balistes capriscus, vary widely (Tab. S3), between 130 mm FL in Iskenderun Bay, Turkey and 230 mm in the Gulf of Mexico, USA (Işmen et al., 2004; Lee, 2019). This is primarily due to different populations of the species distributed throughout the Atlantic. Maturity data should also be assessed from a spatial and temporal perspective. From a spatial perspective, different locations may present different environmental conditions, which can lead to different maturity sizes. From a temporal perspective, density-dependence mechanisms may act on local populations, leading to altered population parameters. Different values at first maturity can also be explained by the fact that some authors may not have used the same maturity stages, which can lead to misinterpretation of some of them (Shinozaki-Mendes et al., 2013b). Despite this and considering other studies carried out on this species, a clear pattern of maturity size changes employing spatial or temporal analyses could not be verified. Temporally, in a study carried out in southeastern USA (Kelly-Stormer et al., 2017), maturity sizes varied inversely for females and males over fifteen years, with females increasing and males decreasing their maturity size. Different studies carried out in the Gulf of Mexico reported maturity sizes with differences of about 50 mm (Fitzhugh et al., 2015; Lee, 2019). In Brazil, a reproductive assessment was carried out with samples collected in the mid-1980s in the state of São Paulo (Bernardes, Dias, 2000). This study, carried out between the southern coast of Espírito Santo and the extreme north of the state of Rio de Janeiro, estimated higher maturity sizes for both males and females compared to the previous 1980s assessment, resulting in large temporal and spatial intervals. Considering that, according to the IUCN, species should be evaluated within three generational intervals (24 years), new studies on gray triggerfish in Brazil were sorely lacking and required.
In some occurrence areas, the gray triggerfish displays socioeconomic importance, as in the Gulf of Mexico and southeastern USA (Johnson, Saloman, 1984; Kelly-Stormer et al., 2017), as well as in southeastern Brazil (Barnardes, Dias, 2000; Hostim-Silva, Soares, 2013). Given this importance, high fishing efforts for the populations of gray triggerfish are evident, which may be responsible for altered population parameters, such as maturity size, due to density-dependence mechanisms. In addition, other factors may also play a role in these changes, such as food web changes due to global warming or decreased gray triggerfish competitors or predators (Kelly-Stormer et al., 2017; Araújo et al., 2022). That being said and considering the commercial importance of the species in the southeastern region and its threatened status, recognizing the estimated maturity size (230 mm FL) reported herein for the purpose of changing the minimum capture size used (200 mm FL) in legislation for the species in Brazil (MMA, 2005) is paramount.
The spawning period of the gray triggerfish, Balistes capriscus, throughout its Atlantic distribution is perhaps the only parameter or characteristic of this species that presents some kind of pattern. Gray triggerfish present reproductive activity patterns associated with increasing temperatures, given that water temperature probably plays an important role in spawning, with accelerated oocyte development potentially taking place in early spring culminating in spawning in mid-spring and early summer, regardless of hemisphere (Bernardes, Dias, 2000; Moore, 2001; Işmen et al., 2004; Kelly-Stormer et al., 2017; Lee, 2019), except for a study carried out in Tunisia where the species was noted as spawning only in summer (Kacem, Neifar, 2014), but still within the range of most studies. A small difference between spawning months is noted among the two studies carried out in Brazil (Bernardes, Dias, 2000; present study). A spawning increase was noted during the summer (November to February) (Bernardes, Dias, 2000; present study), peaking in January in the present study. The hepatosomatic index showed a certain inverse correlation with the gonadosomatic index, indicating that the liver may be correlated with the energy supply for ovarian development during the reproductive period. Considering the estimated spawning periods and the need to establish management measures for the recovery of gray triggerfish in Brazil, we recommend establishing a closed season between November and February of the following years.
In addition to accelerating oocyte development, higher temperatures are also a favorable environmental condition for the survival and growth of gray triggerfish larvae (Kacem, Neifar, 2014). In the study area, higher water temperatures are noted in the summer, when the species begins to spawn and when a high abundance of zooplankton is recorded (Sterza, Fernandes, 2006; Guenther et al., 2008), especially in northern Rio de Janeiro during downwelling, with the predominance of pico- and nanophytoplankton, in addition to high microzooplankton production (Guenther et al., 2008). Similar conditions were observed in the Gulf of Gabes in the Mediterranean for the same species, allowing for the establishment of young individuals that find food resources during this period, which contributes to rapid growth, development and protection (Kacem, Neifar, 2014). Historically, the gray triggerfish is exploited in southeastern Brazil, with a significant decrease in landings recorded in Santa Catarina and with the species being considered overexploited in the states of São Paulo and Espírito Santo (Ataliba et al., 2009; Netto et al., 2009; Hostim-Silva, Soares, 2013; Fraga et al., 2019; Hostim-Silva et al., 2019). Since 2002, management measures have been created to protect the species in Brazil (IBAMA, 2002; MMA, 2005) but, despite this, it is listed as Near Threatened by the Brazilian Biodiversity Extinction Risk Assessment System (IBAMA, 2024). In this sense, we suggest the change of the minimum capture size to 23 cm FL (currently 20 cm) as a recovery and management measure for gray triggerfish fisheries in Southeastern Brazil and, if it is understood that the stocks need further recovery measures, the establishment of a closed season between November and February of the following years along with other possible legislation that acts on fisheries that capture as bycatch during the suggested period (e.g., bottom trawling; Castro et al., 2005; Ataliba, 2009).
Acknowledgments
The authors are grateful to the fishermen for their time and support. We are also grateful to former federal deputy Felipe Rigoni who directed a parliamentary amendment that subsidized the acquisition of equipment necessary for this research.
References
Aggrey-Finn J. The fishery of Balistes capriscus (Balistidae) in Ghana and possible reasons for its collapse. [PhD Thesis]. Bremen: University of Bremen; 2007. Available from: https://webdoc.sub.gwdg.de/ebook/dissts/Bremen/Aggrey2008.pdf
Aggrey-Fynn J. How did fisheries resource Balistes capriscus (Teleostei: Balistidae) disappear in coastal waters of Ghana? J Basic Appl Sci. 2013; 1:45–61.
Aggrey-Fynn J. Distribution and growth of grey triggerfish, Balistes capriscus (Family: Balistidae), in western Gulf of Guinea. West Afr J Appl Ecol. 2009; 15(1):3–11. https://doi.org/10.4314/wajae.v15i1.49421
Araújo BC, Symonds JE, Walker SP, Miller MR. Effects of fasting and temperature on the biological parameters, proximal composition, and fatty acid profile of Chinook salmon (Oncorhynchus tshawytscha) at different life stages. Comp Biochem Physiol A Mol Integr Physiol. 2022; 264:111113. https://doi.org/10.1016/j.cbpa.2021.111113
Ataliba CC, Castro PMG, Carneiro MH. Desembarque do peixe-porco Balistes capriscus capturado pela frota industrial do sudeste e sul do Brasil, com ênfase ao estado de São Paulo. Bol Inst Pesca. 2009; 35(2):247–58.
Bernardes RA, Dias JF. Aspectos da reprodução do peixe-porco, Balistes capriscus (Gmelin) (Actinopterygii, Tetraodontiformes, Balistidae) coletado na costa sul do Estado de São Paulo, Brasil. Rev Bras Zool.2000; 17(3):687–96. https://doi.org/10.1590/S0101-81752000000300014
Bernardes RA. Age, growth and longevity of the gray triggerfish, Balistes capriscus (Gmelin, 1788), from the Southeastern Brazilian Coast. Sci Mar. 2002; 66(2):167–73. https://doi.org/10.3989/scimar.2002.66n2167
Branco ISL, Viana DL, Félix RTS, Véras DP, Hazin FHV. Oocyte development and ovarian maturation of the black triggerfish, Melichthys niger (Actinopterygii: Balistidae) in São Pedro e São Paulo Archipelago, Brazil. Neotrop Ichthyol. 2013; 11:597–606. https://doi.org/10.1590/S1679-62252013000300013
Brown-Peterson NJ. The reproductive biology of spotted sea trout. In: Bortone SA, editor. Biology of the spotted seatrout. Florida: CRC Press; 2003. p.99–133.
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK. A standardized terminology for describing reproductive development in fishes. Mar Coast Fish. 2011; 3(1):52–70. https://doi.org/10.1080/19425120.2011.555724
Burton ML, Potts JC, Carr DR, Cooper M, Lewis J. Age, growth, and mortality of gray triggerfish (Balistes capriscus) from the southeastern United States. Fish Bull. 2015; 113(1):27–39. https://doi.org/10.7755/fb.113.1.3
Castro PMG, Bernardes RA, Carneiro MH, Servo GJM. Balistes capriscus (Gmelin, 1789). In: Cergole MC, Ávila-da-Silva AO, Rossi-Wongtschowski CLDB, editors. Análise das principais pescarias comerciais da região Sudeste-Sul do Brasil: dinâmica populacional das espécies em explotação. São Paulo: Série documentos Revizee – Score Sul; 2005. p.29–34. Available from: https://www.marinha.mil.br/secirm/sites/www.marinha.mil.br.secirm/files/documentos/revizee/score-sul-3.pdf
Darling ES, Graham NAJ, Januchowski-Hartley FA, Nash KL, Pratchett MS, Wilson SK. Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs. 2017; 36:561–75. https://doi.org/10.1007/s00338-017-1539-z
Eduardo LN, Bertrand A, Frédou T, Lira AS, Lima RS, Ferreira BP et al. Biodiversity, ecology, fisheries, and use and trade of Tetraodontiformes fishes reveal their socio‐ecological significance along the tropical Brazilian continental shelf. Aquat Conserv. 2020; 30(4):761–74. https://doi.org/10.1002/aqc.3278
Fitzhugh GR, Lyon HM, Barnett BK. Reproductive parameters of gray triggerfish (Balistes capriscus) from the Gulf of Mexico: sex ratio, maturity and spawning fraction. SEDAR43-WP-03; 2015.
Fonteles-Filho AA. Oceanografia, biologia e dinâmica populacional de recursos pesqueiros. Fortaleza: Expressão Gráfica; 2011.
Fraga C, Peixoto A, Leite Y, Santo N, Oliveira J, Sylvestre L et al. Lista da fauna e flora ameaçadas de extinção no estado do Espírito Santo. In: Fraga CN, Formigoni MH, Chaves FG, editors. Fauna e flora ameaçadas de extinção no estado do Espírito Santo. Santa Teresa, Instituto Nacional da Mata Atlântica; 2019. p.342–419.
Fricke R, Eschmeyer WN, Van der Laan R. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2018. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Guenther M, Gonzalez-Rodriguez E, Carvalho WF, Rezende CE, Mugrabe G, Valentin JL. Plankton trophic structure and particulate organic carbon production during a coastal downwelling-upwelling cycle. Mar Ecol Progr Ser. 2008; 363:109–19. https://doi.org/10.3354/meps07458
Hernández JMR, Shervette VR. Queen triggerfish Balistes vetula age-based population demographics and reproductive biology for waters of the North Caribbean. Fishes. 2024; 9(5):162. https://doi.org/10.3390/fishes9050162
Hilborn R, Walters CJ. Quantitative fisheries stock assessment: choice, dynamics and uncertainty. New York: Springer; 1992.
Hood PB, Johnson AK. A study of the age structure, growth, maturity schedules and fecundity of gray triggerfish (Balistes capriscus), red porgy (Pagrus pagrus), and vermilion snapper (Rhomboplites aurorubens) from the Eastern Gulf of Mexico. Florida: MARFIN Final Report; 1997.
Hostim-Silva M, Soares SSG. Boletim estatístico da pesca do Espírito Santo-Ano 2011. Programa de estatística pesqueira do Espírito Santo. Vitória: Universidade Federal do Espírito Santo; 2013. Available from: https://www.icmbio.gov.br/cepsul/images/stories/biblioteca/download/estatistica/es/est_2011_es_boletim_estatistico_pesca_1.pdf
Hostim-Silva M, Duboc LF, Pimentel CR, Vilar CC, Machado DF, Di Dario F et al. Peixes ameaçados de extinção no estado do Espírito Santo. In: de Fraga CN, Formigoni MH; Chaves FG, editors. Fauna e flora ameaçadas de extinção no estado do Espírito Santo. Santa Teresa: Instituto Nacional da Mata Atlântica; 2019. p.230–55.
Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA). Portaria 81/2002. Proíbe o emprego do puçá nas regiões Sudeste e Sul do país [Internet]. Brasília; 2002. Available from: www.ibama.gov.br/sophia/cnia/legislacao/IBAMA/PT0081-100702.PDF
Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA). Sistema de informação sobre a biodiversidade brasileira. Balistes capriscus. Ano de avaliação: 2014 [Internet] IBAMA; 2024. Available from: https://specieslist.homologacao.sibbr.gov.br/speciesListItem/list/drt1572557301581?q=Balistes+capriscus
Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Sistema de Avaliação do Risco de Extinção da Biodiversidade – SALVE [Internet] ICMBio; 2024. Available from: https://salve.icmbio.gov.br/
Ingram GW. Stock structure of gray triggerfish, Balistes capriscus, on multiple spatial scales in the Gulf of Mexico. [PhD Thesis]. Alabama: University of South Alabama; 2001.
Işmen A, Türkoglu M, Yigin CÇ. The age, growth and reproduction of gray triggerfish. Pak J Biol Sci. 2004; 7(12):2135–38. https://doi.org/10.3923/pjbs.2004.2135.2138
International Union for Conservation of Nature (IUCN). Guidelines for using the IUCN red list categories and criteria. The IUCN Red List of Threatened Species 122; 2024. Available from: https://www.iucnredlist.org/
Johnson AG, Saloman CH. Age, growth, and mortality of gray triggerfish, Balistes capriscus, from the northeastern Gulf of Mexico. Fish Bull. 1984; 82(3):485–92.
Junqueira LC, Carneiro J. Histologia básica. Rio de Janeiro: Guananbara Koogan; 2013.
Kacem H, Neifar L. The reproductive biology of the grey triggerfish Balistes capriscus (Pisces: Balistidae) in the Gulf of Gabès (south-eastern Mediterranean Sea). J Mar Biol Assoc U K. 2014; 94:1531–37. https://doi.org/10.1017/S0025315414000824
Kacem H, Boudaya L, Neifar L. Age, growth and longevity of the grey triggerfish, Balistes capriscus Gmelin, 1789 (Teleostei, Balistidae) in the Gulf of Gabès, southern Tunisia, Mediterranean Sea. J Mar Biol Assoc U K. 2015; 95:1061–67. https://doi.org/10.1017/S0025315414002148
Kelly-Stormer A, Shervette V, Kolmos K, Wyanski D, Smart T, McDonough C et al. Gray triggerfish reproductive biology, age, and growth off the Atlantic Coast of the Southeastern USA. Trans Am Fish Soc. 2017; 146:523–38. https://doi.org/10.1080/00028487.2017.1281165
King M. Fisheries biology, assessment and management. Hoboken: John Wiley & Sons; 2013.
Lang ET, Fitzhugh GR. Oogenesis and fecundity type of gray triggerfish in the Gulf of Mexico. Mar Coast Fish. 2015; 7:338–48. https://doi.org/10.1080/19425120.2015.1069428
Lee AM. A Snapshot of the age, growth, and reproductive status of gray triggerfish (Balistes Capriscus, Gmelin 1789) on three artificial reefs in the northwest Gulf ofMexico. [Master Dissertation]. Texas: The University of Texas Rio Grande Valley; 2019.
Liu J, Zapfe G, Shao K-T, Leis JL, Matsuura K, Hardy G et al. Balistes capriscus (errata version published in 2016). The IUCN Red List of Threatened Species 2015: e.T193736A97662794. 2015.
Lobel PS, Johannes RE. Nesting, eggs and larvae of triggerfishes (Balistidae). Environ Biol Fishes. 1980; 5:251–52. https://doi.org/10.1007/BF00005359
Matsuura K. Taxonomy and systematics of tetraodontiform fishes: a review focusing primarily on progress in the period from 1980 to 2014. Ichthyol Res. 2014; 62:72–113. https://doi.org/10.1007/s10228-014-0444-5
McBride RS, Somarakis S, Fitzhugh GR, Albert A, Yaragina NA, Wuenschel MJ et al. Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish Fish. 2015; 16(1):23–57. https://doi.org/10.1111/faf.12043
Monteiro-Neto C, Cunha FEA, Nottingham MC, Araújo ME, Rosa IL, Barros GML. Analysis of the marine ornamental fish trade at Ceará State, northeast Brazil. Biodiv Conserv. 2003; 12:1287–95. https://doi.org/10. 1023/A:1023096023733
Moore JL. Age, growth and reproductive biology of the gray triggerfish (Balistes capriscus) from the southeastern United States, 1992-1997. [Master Dissertation]. South Carolina: University of Charleston; 2001.
Ministério do Meio Ambiente (MMA). Instrução Normativa MMM n°53 de 22 de novembro de 2005 [Internet]. Brasília: MMA; 2005. Available from: https://www.icmbio.gov.br/cepsul/images/stories/legislacao/Portaria/2005/in_mma_53_2005_altrda_tamanho_minimo_especies_marinhas_estuarinas_se_s_altrd_in_mma_03_2006.pdf
Netto R, Krohling W, Rocha MB, Di Beneditto APM. Produção pesqueira no triênio 2003-2005 pela cooperativa de pesca de Vila Velha, Espírito Santo, sudeste do Brasil. Bol Inst Pesca. 2009; 35(4):663–73.
Nóbrega MF, Júnior JG, Oliveira JEL. Peixes da pesca artesanal. Rio de Janeiro: Museu Nacional; 2015.
Ogle DH, Doll JC, Wheeler AP, Dinno A. FSA: simple fisheries stock assessment methods. 2023. R package version 0.9.5. Available from: https://cran.r-project.org/package=FSA
Patankar V, Paranjape A, Tyabji Z, Wagh T, Marathe A. Occurrence and distribution of tetraodontiform fishes of the Andaman and Nicobar Islands. Check List. 2014; 14(3):529–37. https://doi.org/10.15560/14.3.529
R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2024. Available from: https://www.r-project.org/
Santini F, Sorenson L, Alfaro ME. A new phylogeny of tetraodontiformes fishes (Tetraodontiformes, Acanthomorpha) based on 22 loci. Mol Phylog Evol. 2013; 69(1):177–87. https://doi.org/10.1016/j.ympev.2013.05.014
Southeast Data, Assessment, and Review (SEDAR). Stock assessment report of SEDAR 9: Gulf of Mexico gray triggerfish. South Carolina: SEDAR, North Charleston; 2006. Available from: https://sedarweb.org/documents/sedar-09-stock-assessment-report-gulf-of-mexico-gray-triggerfish/
Shinozaki-Mendes RA, Santander-Neto J, Silva JRF, Hazin FHV. Gonad maturation of Haemulon plumieri (Teleostei: Haemulidae) in Ceará state, Northeastern Brazil. Braz J Biol. 2013a; 73(2):383–90. https://doi.org/10.1590/S1519-69842013000200019
Shinozaki-Mendes RA, Santander-Neto J, Silva JRF, Hazin FHV. Reproductive biology of Haemulon plumieri (Teleostei: Haemulidae) in Ceará state, northeastern Brazil. Braz J Biol. 2013b; 73(2):391–96. https://doi.org/10.1590/S1519-69842013000200020
Simmons CM, Szedlmayer ST. Territoriality, reproductive behavior, and parental care in gray triggerfish, Balistes capriscus, from the northern Gulf of Mexico. Bull Mar Sci. 2012; 88(2):197–209. http://dx.doi.org/10.5343/bms.2011.1012
Sparre P, Venema SC. Introdução à avaliação de mananciais de peixes tropicais. Parte 1. Manual. Roma: Organização das Naçães Unidas para a Alimentação e a Agricultura; 1997.
Sterza JM, Loureiro FL. Zooplankton community of the Vitória bay estuarine system (Southeastern Brazil): characterization during a three-year study. Braz J Oceanogr. 2006; 54:95–105. https://doi.org/10.1590/S1679-87592006000200001
Stump E, Ralph GM, Comeros-Raynal MT, Matsuura K, Carpenter KE. Global conservation status of marine pufferfishes (Tetraodontiformes: Tetraodontidae). Glob Ecol Conserv. 2018; 14:e00388. https://doi.org/10.1016/j.gecco.2018.e00388
Tyler JC. Osteology, phylogeny, and higher classification of the fishes of the order Plectognathi (Tetraodontiformes). Seattle: U.S. Dept. of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service; 1980.
Vianna M, Rodrigues AMT, Lin CF. Descrição da pescaria de peroá (Balistes capriscus) com a utilização do puçá-grande no sudeste do Brasil. Bol Inst Pesca. 2007; 33(2):229–36.
Wallace RA, Selman K. Cellular and dynamic aspects of oocyte growth in teleosts. Am Zool. 1981; 21(2):325–43.
White DB, Wyanski DM, Sedberry GR. Age, growth, and reproductive biology of the blackbelly rosefish from the Carolinas, USA. J Fish Biol. 1998; 53(6):1274–91. https://doi.org/10.1111/j.1095-8649.1998.tb00248.x
Wilson CA, Nieland DL, Stanley AL. Age, growth, and reproductive biology of gray triggerfish (Balistes capriscus) from the northern Gulf of Mexico commercial harvest. Gulf of Mexico; 1995. MARFIN Final Report.
Authors
![]() Jones Santander-Neto1
Jones Santander-Neto1 ![]() ,
, ![]() Nádia Ortolan da Vitória1,
Nádia Ortolan da Vitória1, ![]() Henrique David Lavander2 and
Henrique David Lavander2 and ![]() Andressa C. M. de Melo1
Andressa C. M. de Melo1
[1] Laboratório de Dinâmica de Populações Marinhas, Instituto Federal do Espírito do Espírito Santo, Rua Augusto da Costa Oliveira, 660, Praia Doce, 29285-000 Piúma, ES, Brazil. (JSN) jones.santander@ifes.edu.br (corresponding author), (NOV) nadia.engepesca@gmail.com, (ACMM) andressa_cmm@hotmail.com.
[2] Malacolab, Rua Augusto da Costa Oliveira, 660, Praia Doce, 29285-000 Piúma, ES, Brazil. (HDL) henrique.lavander@ifes.edu.br
Authors’ Contribution 

Jones Santander-Neto: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing-original draft, Writing-review and editing.
Nádia Ortolan da Vitória: Data curation, Formal analysis, Investigation, Software, Writing-original draft.
Henrique David Lavander: Data curation, Formal Funding acquisition, Writing-review and editing.
Andressa C. M. de Melo: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing-original draft, Writing-review and editing.
Ethical Statement
Not applicable.
Competing Interests
The author declares no competing interests.
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Funding
This work was supported by Fundação de Amparo à Pesquisa e Inovação do Espírito Santo – FAPES (Project information DI 003/2022 – SEAG/FAPES – PPEDAGRO) and SubSea7.
How to cite this article
Santander-Neto J, Vitória NO, Lavander HD, Melo ACM. Reproductive biology of the gray triggerfish Balistes capriscus (Tetraodontiformes: Balistidae) in southeastern Brazil as a tool for fisheries management. Neotrop Ichthyol. 2025; 23(3):e240137. https://doi.org/10.1590/1982-0224-2024-0137
Copyright
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Distributed under
Creative Commons CC-BY 4.0

© 2025 The Authors.
Diversity and Distributions Published by SBI
![]() Accepted June 16, 2025
Accepted June 16, 2025
![]() Submitted February 13, 2025
Submitted February 13, 2025
![]() Epub September 29, 2025
Epub September 29, 2025