![]() Francisco de Menezes Cavalcante Sassi1,
Francisco de Menezes Cavalcante Sassi1, ![]() Marcelo de Bello Cioffi1
Marcelo de Bello Cioffi1 ![]() and
and ![]() Orlando Moreira-Filho1
Orlando Moreira-Filho1
PDF: EN XML: EN | Cite this article
Abstract
Loricariidae is a Neotropical fish family divided into six subfamilies and ranking in third among the most biodiverse fish groups. This study conducts an updated review of the cytogenetic investigations within the family, discussing the trends in chromosomal evolution and the main gaps and future directions for studies. Covering 125 publications that analyzed 234 species from all subfamilies except Lithogeninae, corresponding to about 21% of the valid species diversity, our study revealed samples from six different river basins in Argentina, Brazil, Ecuador, Paraguay, and Venezuela. There was a dearth of data for northeast Brazil, the Western Amazon, the Guianas Shield, and other Neotropical countries. In loricariids, there are seven different sex chromosome systems and a variety of diploid numbers (2n) ranging from 33 to 96 as a result of different chromosomal rearrangements such as fusions, fissions, translocations, and inversions. We recorded more simple nucleolar organizer regions (Ag-NOR) compared to multiple ones, and the fundamental number (FN) varied between 34 and 142. Populational studies have been conducted only in a few taxa, but a remarkable karyotype variation that includes B chromosomes is shown. Despite continuous efforts, cytogenetics still does not adequately capture the diversity of Loricariidae.
Keywords: Ag-NOR, B chromosomes, Karyotype, Plecos, Sex chromosomes.
Loricariidae é uma família de peixes Neotropicais dividida em seis subfamílias e ranqueada na terceira posição dentre os grupos mais biodiversos de peixes. Este estudo conduz uma revisão atualizada das investigações citogenéticas na família, discutindo as tendências evolutivas cromossômicas e as principais lacunas e direções futuras para estudos. Cobrindo 125 publicações que analisam 234 espécies de todas as subfamílias, exceto Lithogeninae, correspondendo a aproximadamente 21% da diversidade de espécies válidas, nosso estudo revelou amostragens em seis bacias hidrográficas distintas na Argentina, Brasil, Equador, Paraguai e Venezuela. Há uma escassez de dados para o nordeste do Brasil, o oeste da Amazônia, o escudo das Guianas e outros países Neotropicais. Em loricariídeos, há sete sistemas de cromossomos sexuais distintos e uma variedade de números diploides (2n), variando de 33 a 96 como resultado de rearranjos cromossômicos diferentes como fusões, fissões, translocações e inversões. Nós registramos mais regiões organizadoras de nucléolo (Ag-RON) simples quando comparado com múltiplas, e o número fundamental (NF) variou entre 34 e 142. Estudos populacionais foram realizados em poucos organismos, mas uma extraordinária variação cariotípica que inclui cromossomos B é demonstrada. Apesar de esforços contínuos, a citogenética ainda não captura adequadamente a diversidade de Loricariidae.
Palavras-chave: Ag-RON, Cariótipo, Cascudos, Cromossomos B, Cromossomos sexuais.
Introduction
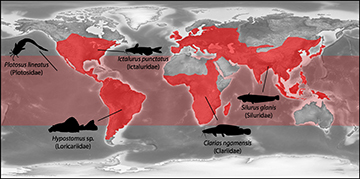
Among vertebrates, the paraphyletic group of fish outstands because of its high richness and diversity of morphology and ecology (Nelson et al., 2016). They can be found all over the Earth in freshwater and marine environments, and humans exploit them widely as primary food sources or as ornamental species for the aquarium trade (Evers et al., 2019; Novák et al., 2020, 2022). Such diversity is observed in about 36,000 valid species, with almost half of them belonging to the 450 families that make up the infraclass Teleostei (Fricke et al., 2023). More than 18,000 species are found in freshwater environments, the majority of which are found in tropical and subtropical regions, including the Amazon River Basin, tropical Africa, and southeast Asia (Lévêque et al., 2008; Nelson et al., 2016). The monophyletic order Siluriformes (popularly known as catfishes), is home to a large portion of this variety (Fink, Fink, 1981, 1996; Arratia et al., 2003; Saitoh et al., 2003; Sullivan et al., 2006). This order is composed by the suborder Loricarioidei, which consists of six families that are endemic to South America, and Siluroidei, which includes 29 families that are spread over all continents except Antarctica (Sullivan et al., 2006). Due to their widespread distribution (Fig. 1), research focusing on the biogeography of siluriform fishes often indicates that South America may have served as the group’s center of origin (Briggs, 2005; Sullivan et al., 2006).
FIGURE 1| World map showing the distribution of Siluriformes (red areas) according to Nelson et al. (2016) and GBIF (2023), with illustrations of important families in distinct regions.
Loricariidae species, which rank third among the most biodiverse fish families, are known by several names, including armored catfishes, plecos, “cascudos”, “bodós”, and “acaris”. Together with Characidae and Cyprinidae, it corresponds for about 50% of all Ostariophysi species (Nelson et al., 2016). According to Fricke et al. (2023), 1,051 valid species are divided into six subfamilies: Delturinae, Hypoptopomatinae, Hypostominae, Lithogeninae, Loricariinae, and Rhinelepinae. Their distinct anatomy, which includes a suckermouth positioned ventral and dermal plates covering the body, allows them to be recognized despite their widespread distribution in the Neotropical region. Loricariids are iliophagous, with a vast diversity of feeding habitats ranging from wood to detritus and algae (Buck, Sazima, 1995; Lujan et al., 2017). They demonstrate a broad range of sizes, from less than 5 cm (e.g., Ribeiro et al., 2012) to approximately 1 m (Lujan et al., 2010). Given a combination of excessive reaping for the aquarium trade and the destruction of their habitats, some loricariids are threatened (Barros et al., 2023). Indeed, the high ornamental trade of plecos led to the development of the L-code (or L-number). This code was developed by aquarists, aiming to create a temporary “voucher number” for morphological variants identified by aquarium trade that lack a proper taxonomic identification or are difficult to classify only by morphological features (Stawikowski et al., 2004). Recently, 223 Loricariidae species have been assigned from their L-numbers to valid species name (Novák et al., 2022).
The description of fish species is often complex. While some species present a conservation of morphological features, body-color variation and polymorphisms are frequently observed in fish (Price et al., 2008). In this context, the use of complementary methods is fundamental to assessing all biodiversity, in especial cytogenetics (Stace, 2000), the field of genetics that studies chromosomes from their structure and molecular composition to their organization and variation among species. Cytogenetics is an important tool for discovering the Neotropical fish biodiversity, as exemplified by the outstanding number of new and/or cryptic species in addition to karyomorphs with absence of hybrid forms reported (e.g., Pazian et al., 2012; Ferreira et al., 2014; Oliveira et al., 2016; Ramirez et al., 2017; Rocha-Reis et al., 2018). Among loricariids, Artoni, Bertollo (2001) suggest that 2n = 54 might represent the basal diploid number for this family, with distinct trends in evolution among its subfamilies. Considering the continuous growing number of studies involving Loricariidae species, here we review for the first time the scientific literature that includes cytogenetic data for Loricariidae species. We discussed the trends in chromosomal evolution in this representative fish group and the main gaps and future directions for studies.
Material and methods
A detailed search was conducted on Google Scholar until December of 2023, using the keywords “Cytogenetics Loricariidae” and “Chromosomes Loricariidae”. During the screening of research papers, all the duplicate papers and cytogenetic papers without karyotype information were excluded from our compilation (for example Traldi et al., 2019, where only mapping of repetitive sequences was conducted, without addressing the diploid number — 2n and karyotype structure). We included all papers found published between 1968 and 2023 in journals in any language available. For more assurance on the quality of results, the data analyzed was double-checked by the authors: the resulted table comprising the cytogenetic features was checked by each author, comparing with the original papers to determine whether such articles would comply with the inclusion and exclusion criteria. We followed the classification of chromosomes by the centromeric position and arm ratios, proposed by Levan et al. (1964) and standardized for fish in Arai (2011) as metacentric (m), submetacentric (sm), subtelocentric (st) and acrocentric (a).
From each paper, we extracted cytogenetic and biogeographic data such as diploid number (2n), fundamental number (NF), karyotype formula (KF), sex chromosome system (SCS) and AgNOR/18S rDNA sites (simple or multiple). Additionally, locality and geographical coordinates of the populations investigated were also compiled, with the city name, state, and country indicated according to the coordinates provided in the papers. Species and family names were checked against Eschmeyer’s Catalog of Fishes (Fricke et al., 2023) and updated according to the valid names. Taxonomic abbreviations were maintained as present in the papers, following: “sp.” for specimens not fully identified, “prope” for specimens with resemblance to a described taxon but not identical, “aff.” for closely related to an identified taxon or with uncertainty in taxonomic identification, “cf.” for specimens similar to or comparable to a taxon but with doubts, “n.sp.” for proposed new species, and “L.” for locally variants of specimens. For the papers in which the geographical coordinates were available, we plotted those sample points into maps using QGIS 3.28.2.
Results
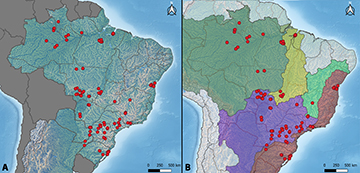
We compiled 125 papers that included cytogenetic data on Loricariidae species. These studies comprised a diversity of 234 species, here included those identified as “sp.”, prope, “aff.”, “cf.”, “n.sp.”, and “L” as distinct species, from 48 genus. Hypostominae was the subfamily most represented with 142 species karyotyped, followed by Loricariinae (54 spp.), Hypoptopomatinae (34 spp.), and Delturinae and Rhinelepinae with 2 spp. each, with absence of data for Lithogeninae. The Hypostomus genus was the most represented with 26 valid species karyotyped, in a total of 159 records when including all populational data and those identified as “sp.”, prope, “aff.”, “cf.”, “n. sp.”, and “L”. The geographical coordinates plotted into maps show a distribution of species cytogenetically investigated in Brazil, Argentina, and Paraguay, comprising six distinct river basins (Fig. 2). Although species from Ecuador and Venezuela were also compiled, those papers do not present geographical coordinates of the sample sites.
FIGURE 2| Partial maps of South America showing the distribution of Loricariidae species (red dots) investigated by cytogenetics that have the geographical coordinates published together. A. Map highlighting the geopolitical regions of South America, with emphasis on Brazil, Paraguay, and part of Argentina territory; B. Map highlighting the hydrographic basins of South America, with emphasis on Amazonas (green), Tocantins-Araguaia (yellow), Paraná/Paraguay (purple), São Francisco (cyan), Atlantic East and Atlantic South (red) River Basins.
Diploid number varied from 2n = 33 in males of Rineloricaria teffeana (Steindachner, 1879) (Marajó et al., 2022) to 2n = 96 in Hemipsilichthys sp. (Kavalco et al., 2004, 2005). The fundamental number ranged from 34 in R. teffeana (Marajó et al., 2022) to 142 in Hypostomus topavae (Godoy, 1969) (Kamei et al., 2017), and the simple distribution of Ag-NOR/18S rDNA was the most common with 269 records, against 110 records of multiple sites. Seven sex chromosomes systems were described for 31 Loricariidae species, the simple XX/XY, XX/X0, and ZZ/ZW, and the multiples X1X1X2X2/X1X2Y, XX/XY1Y2, ZZ/ZW1W2, and Z1Z1Z2Z2/Z1Z2W1W2. B chromosomes were found in five species, varying from 1B (e.g., de Souza et al., 2009) to 3B chromosomes (e.g., Porto et al., 2010). Complete results were compiled in Tab. 1.
TABLE 1 | Review of cytogenetic data by subfamily of Loricariidae published as articles. Geographical coordinates ending with asterisk indicates mismatched coordinates. Diploid number (2n), fundamental number (NF), karyotype formula (KF), sex chromosome system (SCS) are displayed.
Subfamily/Genus | Species | Locality | Latitude | Longitude | Basin | 2n | NF | Ag-NOR – 18S | KF | SCS |
Reference/ |
Loricariinae | |||||||||||
Brochiloricaria | macrodon (Kner, 1853) |
|
|
|
| 58♀♂ | 78 |
| 18m + 2sm + 38st/a |
| Michele et al. (1977). Reported as Loricaria macrodon |
Farlowella | amazonum (Günther, 1864) | Agua Boa stream, Mundo Novo, MS, Brazil |
|
|
| 58♀♂ | 110 | Simple | 6m + 38sm + 8st + 6a |
| Fernandes et al. (2012) |
| amazonum | Dourado stream, Japorã, MS, Brazil | 23°51’04.9"S | 54°09’51.1"W | Iguatemi – Paraná | 58♀♂ | 110 | Simple | 12m + 30sm + 10st + 6a |
| Fernandes et al. (2015) |
| cf. amazonum | Paraná do Piloto, Barcelos, AM, Brazil | 0°56’04.8"S | 62°58’01.6"W | Negro – Amazonas | 58♀♂ | 116 | Simple | 14m + 30sm + 14st |
| Marajó et al. (2018) |
| schreitmuelleri Arnold, 1936 | Jundiá stream, Manaus, AM, Brazil | 2°19’43.8"S | 60°04’40.4"W | Cuieiras – Amazonas | 58♀♂ | 112 | Simple | 10m + 30sm + 14st + 4a |
| Marajó et al. (2018) |
| hahni Meinken, 1937 | Dourado stream, Mundo Novo, MS, Brazil | 23°51’04.9"S | 54°26’31.4"W | Paraná | 58♀♂ | 110 | Simple | 12m + 30sm + 10st + 6a |
| Fernandes et al. (2021) |
| hahni | Iguaçu River, Capanema, PR, Brazil | 25°38’18.7"S | 54°28’01.7"W | Paraná | 58♀♂ | 112 | Simple | 12m + 20sm + 22st + 4a |
| Fernandes et al. (2021) |
Harttia | carvalhoi Miranda Ribeiro, 1939 | Ribeirão grande, Pindamonhangaba, SP, Brazil | 22°46’03.0"S | 45°26’07.1"W | Paraíba do Sul | 52♀, 53♂ |
| Simple | 18m + 18sm + 8st + 8a or 17m + 18sm + 8st + 10a | XX/XY1Y2 | Centofante et al. (2006) |
| carvalhoi | Pindamonhangaba, SP, Brazil |
|
| Paraíba do Sul | 52♀, 53♂ |
| Simple | 16m + 16sm + 12st + 8a ♀, 15m + 16sm + 12st + 10a ♂ | XX/XY1Y2 | Blanco et al. (2013, 2017); Deon et al. (2022a,b) |
| carvalhoi |
|
|
|
| 52♀♂ |
| Simple | 30m/sm + 20st/a |
| Alves et al. (2003). Reported as Harttia loricariformis, corrected in Centofante et al. (2006) |
| dissidens Rapp Py-Daniel & Oliveira, 2001 | Tambor stream, Rurópolis, PA, Brazil | 4°05’37.8"S | 55°00’30.2"W |
| 54♀♂ | 92 | Simple | 20m + 26sm + 8a |
| Sassi et al. (2021, 2023a,b) |
| duriventris Rapp Py-Daniel & Oliveira, 2001 | Parauapebas River, Canaã dos Carajás, PA, Brazil | 6°30’06.5"S | 50°02’35.3"W | Tocantins-Araguaia | 55♂, 56♀ | 96, 98 | Simple | 16m + 16sm + 16st + 8a ♀, 17m + 16sm + 16st + 6a ♂ | X1X1X2X2/X1X2Y | Sassi et al. (2020, 2023a,b) |
| gracilis Oyakawa, 1993 | Machadinho stream, Santo Antonio do Pinhal, MG, Brazil | 22°48’31.0"S | 45°41’21.0"W | Sapucaí-Mirim | 58♀♂ |
| Simple | 20m + 22sm + 8st + 8a |
| Blanco et al. (2017); Deon et al. (2022a,b) |
| guianensis Rapp Py-Daniel & Oliveira, 2001 | Paraíso stream, Alenquer, PA, Brazil | 1°29’02.2"S | 54°50’31.2"W |
| 58♀♂ | 96 | Simple | 20m + 26sm + 2st + 10a |
| Sassi et al., 2021, 2023a,b |
| intermontana Oliveira & Oyakawa, 2019 | Piranga River, Carandaí, MG, Brazil | 20°59’34.0"S | 43°43’30.0"W |
| 52♀, 53♂ | 90, 91 |
| 14m + 12sm + 12st + 14a ♀, 13m + 12sm + 13st + 15a ♂ | XX/XY1Y2 | Deon et al. (2020, 2022) |
| kronei Miranda Ribeiro, 1908 |
|
|
|
| 58♀♂ |
| Simple | 40m/sm + 18st/a |
| Alves et al. (2003) |
| kronei | Açungui River, Campo Largo, PR, Brazil |
|
| Ribeira | 58♀♂ | 106 | Simple | 14m + 20sm + 14st + 10a |
| Blanco et al. (2013, 2017); Deon et al. (2022a,b) |
| longipinna Langeani, Oyakawa & Montoya-Burgos, 2001 | São Francisco River, Pirapora, MG, Brazil | 17°21’22.3"S | 44°56’59.5"W | São Francisco | 58♀♂ | 102 | Simple | 16m + 12sm + 16st + 14a + 0-2Bs |
| Blanco et al. (2013, 2017); Deon et al. (2022a,b) |
| loricariformis Steindachner, 1877 | Paraitinga River, Silveiras, SP, Brazil | 22°52’22.5"S | 44°51’00.4"W | Paraíba do Sul | 56♀♂ | 106 |
| 16m + 22sm + 10st + 8a |
| Kavalco et al. (2004, 2005); Blanco et al. (2017); Deon et al. (2022a,b) |
| punctata Rapp Py-Daniel & Oliveira, 2001 | Itiquira River, Formosa, GO, Brazil | 15°19’25.0"S | 47°25’26.0"W | Tocantins-Araguaia | 57♂, 58♀ | 116 | Simple | 16m + 20sm + 12st + 10a ♀, 16m + 21sm + 12st + 8a ♂ | X1X1X2X2/X1X2Y | Blanco et al. (2014, 2017); Deon et al. (2022); Sassi et al. (2021, 2023a) |
| rondoni Oyakawa, Fichberg & Rapp Py-Daniel, 2018 | 13 de Maio River, Cachoeira da Serra, PA, Brazil | 8°38’53.0"S | 55°01’41.0"W | Xingu – Amazon | 54♀♂ | 100 | Simple | 20m + 26sm + 4st + 4a | XX/XY | Sassi et al. (2020, 2023a,b) |
| sp. 1 | Macacos stream, Silveiras, SP, Brazil | 22°40’43.0"S | 44°51’25.0"W |
| 56♀, 57♂ | 94 |
| 14m + 14sm + 10st + 18a ♀, 13m + 14sm + 10st + 20a ♂ | XX/XY1Y2 | Deon et al. (2020, 2022a,b) |
| sp. 2 | Barra Grande River, Prudentópolis, PR, Brazil | 24°58’40.7"S | 51°07’34.3"W |
| 62♀♂ | 104 |
| 16m + 14sm + 12st + 20a |
| Deon et al. (2020, 2022a,b) |
| sp. 3 | Rio do Peixe, Cachoeira da Serra, PA, Brazil | 8°39’20.7"S | 55°09’24.1"W |
| 54♀♂ | 96 | Simple | 16m + 18sm + 14st + 6a |
| Sassi et al. (2021, 2023a,b) |
| torrenticola Oyakawa, 1993 | Araras stream, Brazil |
|
| São Francisco | 56♀♂ | 98 | Simple | 16m + 10sm + 16st + 14a |
| Blanco et al. (2012a, 2017); Deon et al. (2022a,b) |
| villasboas Oyakawa, Fichberg & Rapp Py-Daniel, 2018 | Curuá River, Cachoeira da Serra, PA, Brazil | 8°44’09.0"S | 54°57’46.0"W | Xingu – Amazon | 55♂, 56♀ | 96, 98 | Simple | 18m + 24sm + 6st + 8a ♀, 19m + 24sm + 6st + 6a ♂ | X1X1X2X2/X1X2Y | Sassi et al. (2020, 2023a,b) |
Hemiodontichthys | sp. 1 | São Francisco stream, Brazil |
|
| Purus | 46♀♂ |
|
| 42m/sm + 4st/a |
| Carvalho et al. (2018) |
| sp. 2 | Iquiri stream, Brazil |
|
| Purus | 58♀♂ |
|
| 28m/sm + 30st/a |
| Carvalho et al. (2018) |
Loricaria | cataphracta Linnaeus, 1758 | Córrego da Onça, Coxim, MS, Brazil |
|
| Paraguai | 64♀♂ | 86 | Simple and Multiple | 12m + 8sm + 2st + 42a |
| Porto et al. (2014b) |
| cataphracta |
|
|
|
| 64 | 76 |
| 12m/sm + 52st/a |
| Fenocchio et al. (2003). Reported as Loricaria carinata |
| cf. cataphracta | Iguaçu River, Brazil |
|
| Paraná | 64♀♂ |
| Simple | 12m + 8sm + 2st + 42a |
| Takagui et al. (2020) |
| simillima Regan, 1904 | Paraná River, Brazil | 27°06’22.5"S | 55°31’20.0"W | Paraná | 64♀♂ | 88 | Simple | 12m + 12sm + 40st/a |
| Benitez et al. (2017) |
| simillima | Miranda River, Brazil |
|
| Paraguai | 64♀♂ |
| Simple | 12m + 8sm + 2st + 42a |
| Takagui et al. (2020) |
Loricariichthys | anus (Valenciennes, 1835) | Capivara stream, Laguna dos Patos system, RS, Brazil | 30°17’34.0"S | 51°19’21.2"W |
| 54♀♂ | 82 | Simple | 10m + 18sm + 26a |
| Takagui et al. (2014) |
| anus | Itapeva Lagoon, Tramandaí system, Brazil | 29°32’11.9"S | 49°55’47.9"W |
| 54♀♂ | 80 | Simple | 10m + 16sm + 28a |
| Takagui et al. (2014) |
| anus | Chimiray stream, Argentina | 28°05’49.4"S | 55°41’37.3"W | Uruguay | 54♀♂ | 82 | Simple | 10m + 18sm + 26a |
| Takagui et al. (2014) |
| anus | Iguaçu River, Brazil |
|
| Paraná | 54♀♂ |
| Simple | 14m + 14sm + 26a |
| Takagui et al. (2020) |
| maculatus (Bloch, 1794) |
|
|
| La Plata | 56 | 78 |
| 22m/sm + 34st/a |
| Fenocchio et al. (2003) |
| platymetopon Isbrücker & Nijssen, 1979 | Jacutinga River, Brazil | 23°13’25.3"S | 50°58’47.4"W | Paranapanema | 54♀♂ | 80 | Simple | 10m + 16sm + 28a |
| Takagui et al. (2014) |
| platymetopon | Paranapanema River, Brazil | 22°42’30.3"S | 51°04’08.4"W |
| 54♀♂ | 80 | Simple | 10m + 16sm + 28a |
| Takagui et al. (2014) |
| platymetopon | Miranda River, Brazil |
|
|
| 54♀♂ |
| Simple | 10m + 16sm + 28a |
| Takagui et al. (2020) |
Pyxiloricaria | menezesi Isbrücker & Nijssen, 1984 | Miranda River, Brazil |
|
| Paraná | 68♀♂ |
| Simple | 8m + 6sm + 2st + 52a |
| Takagui et al. (2020) |
Proloricaria | prolixa (Isbrücker & Nijssen, 1978) | Três Bocas stream, Brazil |
|
| Paraná | 62♀♂ |
| Simple | 20m + 6sm + 36a |
| Takagui et al. (2020) |
Rineloricaria | aequalicuspis Reis & Cardoso, 2001 | Maquine River, RS, Brazil | 29°39’10.4"S | 50°12’31.8"W | Southeast Atlantic | 68♀♂ |
| Simple | 68st/a |
| Venturelli et al. (2021) |
| cadeae (Hensel, 1868) |
|
|
|
| 66♀♂ |
|
| 2m + 64st/a |
| Alves et al. (2003) |
| cadeae | Guaíba Lake, RS, Brazil |
|
|
| 64♀♂ |
| Simple | 2m/sm + 62st/a |
| Maia et al. (2010) |
| cadeae | Forquetinha River, RS, Brazil | 29°24’22.4"S | 52°03’19.2"W |
| 64♀♂ |
| Simple | 64st/a |
| Venturelli et al. (2021) |
| capitonia Ghazzi, 2008 | Uruguai River, São Carlos, SC, Brazil |
|
| Uruguai | 64♀♂ | 70 | Simple | 4m + 2sm + 58st/a |
| Primo et al. (2017) |
| kronei (Miranda Ribeiro, 1911) | Itapocu River, Santa Catarina, Brazil |
|
|
| 64♀♂ |
|
| 6m/sm + 58st/a |
| Alves et al. (2003) |
| lanceolata (Günther, 1868) | Onça Stream, Coxim, MS, Brazil | 18°32’20.0"S | 54°33’43.0"W |
| 48♀♂ | 54 | Multiple | 4m + 2st + 42a |
| Porto et al. (2014a) |
| lanceolata | Onça Stream, Coxim, MS, Brazil | 18°32’20.0"S | 54°33’43.0"W |
| 48♀♂ | 53 | Multiple | 3m + 2st + 43a |
| Porto et al. (2014a) |
| lanceolata | Onça Stream, Coxim, MS, Brazil | 18°32’20.0"S | 54°33’43.0"W |
| 48♀♂ | 55 | Multiple | 3m + 2sm + 2st + 41a |
| Porto et al. (2014a) |
| lanceolata | Onça Stream, Coxim, MS, Brazil | 18°32’20.0"S | 54°33’43.0"W |
| 47♀♂ | 54 | Multiple | 4m + 1sm + 2st + 40a |
| Porto et al. (2014a) |
| lanceolata | Onça Stream, Coxim, MS, Brazil | 18°32’20.0"S | 54°33’43.0"W |
| 47♀♂ | 53 | Multiple | 3m + 1sm + 2st + 41a |
| Porto et al. (2014a) |
| lanceolata | Onça Stream, Coxim, MS, Brazil | 18°32’20.0"S | 54°33’43.0"W |
| 47♀♂ | 52 | Multiple | 2m + 1sm + 2st + 42a |
| Porto et al. (2014a) |
| lanceolata | Onça Stream, Coxim, MS, Brazil | 18°32’20.0"S | 54°33’43.0"W |
| 46♀♂ | 54 | Multiple | 4m + 2sm + 2st + 38a |
| Porto et al. (2014a) |
| lanceolata | Onça Stream, Coxim, MS, Brazil | 18°32’20.0"S | 54°33’43.0"W |
| 46♀♂ | 53 | Multiple | 3m + 2sm + 2st + 39a |
| Porto et al. (2014a) |
| lanceolata | Onça Stream, Coxim, MS, Brazil | 18°32’20.0"S | 54°33’43.0"W |
| 46♀♂ | 52 | Multiple | 2m + 2sm + 2st + 40a |
| Porto et al. (2014a) |
| lanceolata | Onça Stream, Coxim, MS, Brazil | 18°32’20.0"S | 54°33’43.0"W |
| 45 | 53 |
| 4m + 2sm + 37a |
| Porto et al. (2014a) |
| latirostris (Boulenger, 1900) | Laranjinha River, Ventania, PR, Brazil |
|
| Cinzas | 46♀♂ | 60 | Simple | 10m + 4sm + 32st/a |
| Primo et al. (2017) |
| latirostris | Barra Grande River, Prudentópolis, PR, Brazil |
|
| Ivaí | 46♀♂ | 60 | Simple | 10m + 4sm + 32st/a |
| Primo et al. (2017) |
| latirostris | Das Pedras River, Ventania, PR, Brazil | 24°07’39.29"S | 50°14’16.21"W |
| 46♀♂ | 60 | Simple | 10m + 4sm + 32st/a |
| Glugoski et al. (2018) |
| latirostris | Piumhi River, Piumhi, MG, Brazil | 20°31’55"S | 46°02’42"W |
| 48♀♂ | 60 | Simple | 6m + 6sm + 36st/a |
| Glugoski et al. (2018) |
| latirostris | Laranjinha River, Ventania, PR, Brazil | 23°58’13"S | 50°14’01"W |
| 46♀♂ | 60 | Simple | 10m + 4sm + 32st/a |
| Glugoski et al. (2022) |
| latirostris | Piumhi River, Piumhi, MG, Brazil | 20°31’55"S | 46°02’42"W |
| 48♀♂ | 60 | Simple | 6m + 6sm + 36st/a |
| Glugoski et al. (2022) |
| lima (Kner, 1853) | Ribeira River, Areia stream, or Açungui River Ponta Grossa, PR, Brazil |
|
| Paraná | 70♀♂ | 72 | Simple | 2st + 68a |
| Rosa et al. (2012) |
| lima | Ribeira River, Areia stream, or Açungui River Ponta Grossa, PR, Brazil |
|
| Paraná | 70♀♂ | 74 | Simple | 2m + 2st + 66a |
| Rosa et al. (2012) |
| lima | Ribeira River, Areia stream, or Açungui River Ponta Grossa, PR, Brazil |
|
| Paraná | 69♀♂ | 72 | Simple | 1m + 2st + 66a |
| Rosa et al. (2012) |
| lima | Ribeira River, Areia stream, or Açungui River Ponta Grossa, PR, Brazil |
|
| Paraná | 69♀♂ | 73 | Simple | 1m + 1sm + 2st + 65a |
| Rosa et al. (2012) |
| lima | Ribeira River, Areia stream, or Açungui River Ponta Grossa, PR, Brazil |
|
| Paraná | 68♀♂ | 72 | Simple | 2m + 2st + 64a |
| Rosa et al. (2012) |
| lima | Ribeira River, Areia stream, or Açungui River Ponta Grossa, PR, Brazil |
|
| Paraná | 68♀♂ | 74 | Simple | 1m + 1m + 2sm + 2st + 62a |
| Rosa et al. (2012) |
| lima | Ribeira River, Areia stream, or Açungui River Ponta Grossa, PR, Brazil |
|
| Paraná | 66♀♂ | 72 | Simple | 2m + 1m + 1m + 2st + 60a |
| Rosa et al. (2012) |
| lima | Ribeira River, Areia stream, or Açungui River Ponta Grossa, PR, Brazil |
|
| Paraná | 66♀♂ | 72 | Simple | 1m + 1m + 1m + 1st + st + 60a |
| Rosa et al. (2012) |
| longicauda Reis, 1983 | Cerquinha Lagoon, RS, Brazil | 30°13’56.5"S | 50°15’40.9"W | Southeast Atlantic | 70♀♂ |
| Simple | 8m/sm + 62st/a |
| Venturelli et al. (2021) |
| malabarbai Rodriguez & Reis, 2008 | Forquetinha River, RS, Brazil | 29°24’22.4"S | 52°03’19.2"W | Southeast Atlantic | 64♀♂ |
| Simple | 4m/sm + 60st/a |
| Venturelli et al. (2021) |
| microlepidogaster (Regan, 1904) | Forquetinha River, RS, Brazil | 29°24’22.4"S | 52°03’19.2"W | Southeast Atlantic | 68♀♂ |
| Simple | 68st/a |
| Venturelli et al. (2021) |
| n. sp. | Betari River, São Paulo, Brazil |
|
|
| 70♀♂ |
|
| 2sm + 68a |
| Alves et al. (2003) |
| parva (Boulenger, 1895) |
|
|
|
| 48♀♂ |
|
|
|
| Hinegardner, Rosen (1972). Reported as Loricaria parva |
| parva | Miranda River, Brazil |
|
| Paraguai | 60♀♂ |
| Simple | 6m/sm + 54st/a |
| Takagui et al. (2020) |
| pentamaculata Langeani & de Araujo, 1994 | Tauá stream, Brazil |
|
| Paraná | 56-59♀♂ | 64-65 |
| 8m/sm + 48st/a or 9m/sm + 47st/a + 0-3Bs |
| Porto et al., 2010 |
| pentamaculata | Jacuaca stream, tributary of Tibagi River, PR, Brazil |
|
|
| 56♀♂ |
|
| 8m/sm + 48st/a |
| Maia et al. (2010) |
| pentamaculata | Água do Oito stream, tributary of Tibagi River, PR, Brazil |
|
|
| 56♀♂ |
|
| 8m/sm + 48st/a |
| Maia et al. (2010) |
| pentamaculata | Tatupeba stream, Brazil |
|
| Paraná | 56♀♂ | 64 | Multiple | 8m/sm + 48st/a |
| Porto et al. (2011) |
| pentamaculata | Tauá stream, Brazil |
|
| Paraná | 56♀♂ | 64-65 | Simple | 8m/sm + 48st/a or 9m/sm + 47st/a |
| Porto et al. (2011) |
| pentamaculata | Keller River, Brazil |
|
| Paraná | 56♀♂ | 64 | Simple | 8m/sm + 48st/a |
| Porto et al. (2011) |
| pentamaculata | Barra Grande River, Prudentópolis, PR, Brazil |
|
| Ivaí | 56♀♂ | 70 | Simple | 4m + 10sm + 42st/a |
| Primo et al. (2017) |
| pentamaculata | Barra Grande River, Prudentópolis, PR, Brazil |
|
| Ivaí | 54♀♂ | 64 | Simple | 6m + 4sm + 44st/a |
| Primo et al. (2018) |
| pentamaculata | Juruba River, Apucarana, PR, Brazil |
|
| Tibagi | 56♀♂ | 70 | Simple | 4m + 10sm + 42st/a |
| Primo et al. (2017) |
| pentamaculata | Itiz stream, Brazil |
|
| Ivaí | 56♀♂ | 64 | Simple | 8m/sm + 48st/a |
| Cius et al. (2022) |
| pentamaculata karyomorph A | Barra Grande river, Prudentópolis, PR, Brazil | 25°05’21.0"S | 50°57’22.0"W | Ivaí | 56♀♂ | 64 | Simple |
|
| Glugoski et al. (2023) |
| pentamaculata karyomorph B | Barra Grande river, Prudentópolis, PR, Brazil | 25°05’21.0"S | 50°57’22.0"W | Ivaí | 55♀♂ | 64 | Simple |
|
| Glugoski et al. (2023) |
| pentamaculata karyomorph C | Barra Grande river, Prudentópolis, PR, Brazil | 25°05’21.0"S | 50°57’22.0"W | Ivaí | 54♀♂ | 64 | Simple |
|
| Glugoski et al. (2023) |
| quadrensis Reis, 1983 | Quadros lagoon, RS, Brazil | 29°44’42.8"S | 50°06’54.3"W | Southeast Atlantic | 70♀♂ |
| Simple | 6m/sm + 64st/a |
| Venturelli et al. (2021) |
| reisi Ghazzi, 2008 | Chimiray stream, Brazil |
|
| Uruguai | 60♀♂ |
| Simple | 60st/a |
| Takagui et al. (2020) |
| stellata Ghazzi, 2008 | Uruguai River, São Carlos, SC, Brazil |
|
| Uruguai | 54♀♂ | 74 | Simple | 6m + 14sm + 34st/a |
| Primo et al. (2017) |
| strigilata (Hensel, 1868) | Forquetinha River, RS, Brazil |
|
| Southeast Atlantic | 68♀♂ |
| Simple | 6m/sm + 62st/a |
| Maia et al. (2010) |
| teffeana (Steindachner, 1879) | Tefé River, Tefé, AM, Brazil | 3°21’14.9"S | 64°40’27.9"W | Amazon | 33♂, 34♀ | 34 | Simple | 1sm + 32a | X1X1X2X2/X1X2Y | Marajó et al. (2022) |
Spatuloricaria | sp. | Xingu River, Altamira, PA, Brazil | 3°19’30.0"S | 52°11’10.0"W | Xingu – Amazon | 66♀♂ | 92 | Simple | 4m + 7sm + 6st + 16a |
| Ferreira et al. (2014) |
| sp. | Caripetuba River, Abaetuba, PA, Brazil | 1°37’23.5"S | 48°55’33.0"W |
| 66♀♂ | 82 |
| 6m + 10sm + 50a |
| Almeida et al. (2023) |
Sturisoma | barbatum (Kner, 1853) | Miranda River, Brazil |
|
| Paraguai | 66♀♂ |
| Simple | 20m + 18sm + 16st + 12a |
| Takagui et al. (2020) |
| cf. nigrirostrum Fowler, 1940 | Araguaia River, Barra do Garças, MT, Brazil |
|
| Tocantins-Araguaia | 74♀♂ |
|
| 20m + 18sm + 36st/a |
| Artoni, Bertollo (2001) |
Hypoptopomatinae | |||||||||||
Corumbataia | cuestae Britski, 1997 | Alambari stream, Botucatu, SP, Brazil |
|
|
| 54♀♂ | 108 | Simple | 34m + 20sm |
| Cristina et al. (2005) |
| cuestae | Lapa stream, SP, Brazil |
|
| Paraná | 54♀♂ |
| Simple | 14m + 10sm + 3st/a |
| Camilo, Moreira Filho (2005) |
| tocantinensis Britski, 1997 | Vermelho River, Goiás, GO, Brazil |
|
|
| 54♀♂ | 108 | Simple | 28m + 26sm |
| Cristina et al. (2005) |
Hisonotus | depressicauda (Miranda Ribeiro, 1918) | Santo Inácio River, Bofete, SP, Brazil |
|
|
| 54♀♂ |
| Simple | 14m + 18sm + 2st + 10a |
| Andreata et al. (1994). Reported as Microlepidogaster depressicauda |
| leucofrenatus (Miranda Ribeiro, 1908) | Poço Grande stream, Juquiá, SP, Brazil |
|
|
| 54-56♀♂ |
| Simple | 24m + 26sm + 4st + 2a | ZZ/ZW | Andreata et al. (1993). Reported as Microlepidogaster leucofrenatus |
| leucofrenatus | Marumbi River, Morretes, PR, Brazil |
|
|
| 54-56♀♂ |
| Simple | 22m + 24sm + 4st + 2a | ZZ/ZW (discarded by Andreata et al., 2010) | Andreata et al. (1993). Reported as Microlepidogaster leucofrenatus |
| leucofrenatus | Cavalo stream, Jaraguá do Sul, SC, Brazil |
|
|
| 54♀♂ |
| Simple | 22m + 24sm + 6st + 2a |
| Andreata et al. (2006) |
| leucofrenatus | Marumbi River, Morretes, PR, Brazil |
|
|
| 54-55♀♂ |
| Simple | 22m + 24sm + 4st + 2a |
| Andreata et al. (2010) |
| leucofrenatus | Cavalo stream, Jaraguá do Sul, SC, Brazil |
|
|
| 54♀♂ |
| Simple | 22m + 24sm + 6st + 2a |
| Andreata et al. (2010) |
| nigricauda (Boulenger, 1891) | Guaíba River, Eldorado do Sul, RS, Brazil |
|
|
| 54♀♂ |
| Simple | 26m + 20sm + 8st |
| Andreata et al. (2006) |
| sp. A | Paraitinga River, Salesópolis, SP, Brazil |
|
|
| 54♀♂ |
| Simple | 26m + 26sm + 2st |
| Andreata et al. (2006) |
| sp. D | Grande stream, Pindamonhangaba, SP, Brazil |
|
|
| 54♀♂ |
| Simple | 26m + 26sm + 2st |
| Andreata et al. (2006) |
Hypoptopoma | inexspectatum (Holmberg, 1893) | Piraí River, Poconé, MT, Brazil |
|
|
| 54♀♂ | 82 | Simple | 10m + 18sm + 8st + 18a |
| Cristina et al. (2005). Reported as Hypoptopoma guentheri |
|
|
|
|
|
|
|
|
|
|
|
|
Isbrueckerichthys | duseni (Miranda Ribeiro, 1907) | Açungui River, Brazil |
|
| Ribeira | 54♀♂ | 108 | Simple | 20m + 20sm + 14st |
| Ziemniczak et al. (2012) |
| duseni | Betari River, Iporanga, SP, Brazil |
|
|
| 54♀♂ |
|
| 20m + 20sm + 14st |
| Alves et al. (2005) |
Kronichthys | lacerta (Nichols, 1919) | Marumbi River, Morretes, PR, Brazil |
|
|
| 54♀♂ |
|
| 20m + 20sm + 14st |
| Alves et al. (2005) |
| lacerta | Marumbi River, Morretes, PR, Brazil |
|
|
| 54♀♂ |
| Simple | 20m + 20sm + 14st |
| Alves et al. (2012a) |
| lacerta | Açungui River, Brazil |
|
| Ribeira | 54♀♂ | 108 | Simple | 22m + 22sm + 10st |
| Ziemniczak et al. (2012) |
| subteres Miranda Ribeiro, 1908 | Betari River, Iporanga, SP, Brazil |
|
|
| 54♀♂ |
|
| 20m + 20sm + 14st |
| Alves et al. (2005) |
Microlepidogaster | sp. A | Alambari River, Botucatu, SP, Brazil |
|
|
| 54♀♂ |
| Multiple | 30m + 20sm + 4st |
| Andreata et al. (1994) |
| sp. B | Móia stream, Penápolis, SP, Brazil |
|
|
| 54♀♂ |
| Simple | 22m + 28sm + 4st |
| Andreata et al. (1994) |
Neoplecostomus | microps (Steindachner, 1877) | Paraitinga River, Brazil | 22°52’22.5"S | 44°51’41.0"W | Paraíba do Sul | 54♀♂ | 108 |
| 24m + 20sm + 10st |
| Kavalco et al. (2004) |
| microps | Paraitinga River, Brazil | 22°52’22.5"S | 44°51’41.0"W | Paraíba do Sul | 54♀♂ | 108 | Simple | 24m + 20sm + 10st |
| Kavalco et al. (2005) |
| microps | Grande stream, Campos do Jordão, SP, Brazil |
|
|
| 54♀♂ |
|
| 20m + 20sm + 14st |
| Alves et al. (2005) |
| microps | Paraíba do Sul River, Brazil |
|
|
| 54♀♂ |
| Simple | 24m + 20sm + 10st |
| Alves et al. (2012a) |
| paranensis Langeani, 1990 | Sapateiro stream, Barbacena, MG, Brazil |
|
|
| 54♀♂ |
|
| 20m + 20sm + 14st |
| Alves et al. (2005) |
| paranensis | Hortelã stream, Botucatu, SP, Brazil |
|
|
| 54♀♂ |
|
| 20m + 20sm + 14st |
| Alves et al. (2005) |
| yapo Zawadzki, Pavanelli & Langeani, 2008 | Verde River, PR, Brazil |
|
| Tibagi | 54♀♂ | 108 | Simple | 18m + 20sm + 16st |
| Ziemniczak et al. (2012) |
Otocinclus | aff. vestitus | Livramento River, Belém, PA, Brazil |
|
|
| 72♀♂ |
| Simple | 22m + 12sm + 4st + 34a |
| Andreata et al. (1994) |
| affinis Steindachner, 1877 | Biguá River, Miracatu, SP, Brazil |
|
|
| 54♀♂ |
| Simple | 46m + 8sm |
| Andreata et al. (1994) |
| affinis | Bonito River, Niterói, RJ, Brazil |
|
|
| 54♀♂ |
| Simple | 40m + 12sm + 12st |
| Andreata et al. (1994) |
| flexilis Cope, 1894 | Santo Antônio da Patrulha, RS, Brazil |
|
|
| 54♀♂ | 108 | Simple | 36m + 18sm |
| Cristina et al. (2005) |
| vittatus Regan, 1904 | Taquari River, Coxim, MS, Brazil |
|
|
| 54♀♂ | 108 | Multiple | 36m + 18sm |
| Cristina et al. (2005) |
| vittatus | Cuiabá River, Santo Antônio do Leverger, MT, Brazil |
|
|
| 54♀♂ | 76 | Simple | 12m + 10sm + 14st + 18a |
| Cristina et al. (2005) |
Otothyris | juquiae Garavello, Britski & Schaefer, 1998 | Rio Preto stream, Itanhaém, SP, Brazil |
|
|
| 54♀♂ | 96 | Simple | 32m + 10sm + 12st |
| Cristina et al. (2005) |
| travassosi Garavello, Britski & Schaefer, 1998 | Ribeira da Terra Firme River, Canavieiras, BA, Brazil |
|
|
| 54♀♂ | 96 | Simple | 26m + 16sm + 12st |
| Cristina et al. (2005) |
Otothyropsis | cf. polyodon | Córrego Dourado, MT, Brazil | 23°51’04.9"S | 54°25’13.9"W |
| 54♀♂ | 108 | Simple | 18m + 28sm + 8st |
| Fernandes et al. (2016) |
Pareiorhaphis | splendens (Bizerril, 1995) | Marumbi River, Morretes, PR, Brazil |
|
|
| 54♀♂ |
|
| 20m + 30sm + 4st |
| Alves et al. (2005). Reported as Hemipsilichthys splendens |
| splendens | Marumbi River, Morretes, PR, Brazil |
|
|
| 54♀♂ |
| Simple | 20m + 20sm + 14st |
| Alves et al. (2012a) |
| splendens | São João River, Garuva, SC, Brazil |
|
|
| 54♀♂ |
|
| 20m + 30sm + 4st |
| Alves et al. (2005). Reported as Hemipsilichthys splendens |
| steindachneri (Miranda Ribeiro, 1918) | Cavalo stream, Jaraguá do Sul, SC, Brazil |
|
|
| 54♀♂ |
|
| 20m + 20sm + 14st |
| Alves et al. (2005). Reported as Hemipsilichthys steindachneri |
| vestigipinnis (Pereira & Reis, 1992) | Caveiras River, Painel, SC, Brazil |
|
|
| 54♀♂ |
|
| 20m + 20sm + 14st |
| Alves et al. (2005). Reported as Hemipsilichthys vestigipinnis |
Pareiorhina | brachyrhyncha Chamon, Aranda & Buckup, 2005 | Ribeirão Grande creek, Pindamonhangaba, SP, Brazil |
|
| Paraíba do Sul | 54♀♂ |
| Simple | 18m + 30sm + 6st |
| Centofante et al. (2011) |
| rudolphi (Miranda Ribeiro, 1911) | Convento stream, Pindamonhangaba, SP, Brazil |
|
|
| 54♀♂ |
|
| 26m + 16sm + 12st |
| Alves et al. (2005) |
| rudolphi | Marimbondo creek, Visconde de Mauá, RJ, Brazil |
|
| Paraíba do Sul | 54♀♂ |
| Simple | 18m + 32sm + 4st |
| Centofante et al. (2011) |
Parotocinclus | maculicauda (Steindachner, 1877) | Açungui River, Brazil |
|
| Ribeira | 54♀♂ | 108 | Simple | 20m + 20sm + 14st |
| Ziemniczak et al. (2012) |
| maculicauda | Poço Grande stream, Juquiá, SP, Brazil |
|
|
| 54♀♂ |
| Simple | 20m + 32sm + 2st |
| Andreata et al. (1994) |
Pseudotocinclus | tietensis (Ihering, 1907) | Tietê River, Paranapiacaba, SP, Brazil |
|
|
| 54♀♂ |
| Simple | 26m + 20sm + 6st | XX/XY | Andreata et al. (1992) |
| n. sp. | Juquiá River, Juquitiba, SP, Brazil |
|
|
| 54♀♂ |
| Simple | 22m + 24sm + 8st |
| Cristina et al. (2005) |
Pseudotothyris | obtusa (Miranda Ribeiro, 1911) | Tributary of Itanhaém River, Itanhaém, SP, Brazil |
|
|
| 54♀♂ |
| Simple | 26m + 18sm + 4st + 6a |
| Andreata et al. (1994) |
Schizolecis | guentheri (Miranda Ribeiro, 1918) | Parati-Mirim stream, Parati, RJ, Brazil |
|
|
| 54♀♂ | 102 | Simple | 30m + 18sm + 6a |
| Cristina et al. (2005) |
| guentheri | Sítio do Meio stream, Mongaguá, SP, Brazil |
|
|
| 54♀♂ | 102 | Simple | 30m + 18sm + 6a |
| Cristina et al. (2005) |
| guentheri | Descoberto stream, Guaratuba, PR, Brazil |
|
|
| 54♀♂ | 102 | Simple | 30m + 18sm + 6a |
| Cristina et al. (2005) |
| guentheri | Garuva River, Garuva, SC, Brazil |
|
|
| 54♀♂ | 102 | Simple | 30m + 18sm + 6a |
| Cristina et al. (2005) |
Hypostominae | |||||||||||
Ancistomus | spilomma (Cardoso & Lucinda, 2003) | Araguaia River, Pontal do Araguaia, MT, Brazil | 15°50’15.0"S | 51°58’43.0"W |
| 52♀♂ |
| Multiple | 25m + 21sm + 6st ♀, 24m + 22sm + 6st ♂ | ZZ/ZW | Oliveira et al. (2006). Reported as Hemiancistrus spilomma |
| spinosissimus (Cardoso & Lucinda, 2003) | Araguaia River, Pontal do Araguaia, MT, Brazil | 15°50’15.0"S | 51°58’43.0"W |
| 52♀♂ |
| Simple | 26m + 22sm + 4st |
| Oliveira et al. (2006). Reported as Hemiancistrus spinosissimus |
| feldbergae | PA, Brazil |
|
|
| 52♀♂ |
| Simple | 38m/sm + 14st |
| Pety et al. (2018). Reported as Peckoltia feldbergae |
Ancistrus | abilhoai Bifi, Pavanelli & Zawadzki, 2009 | Iguaçu River, União da Vitória, PR, Brazil | 26°15’64”S* | 51°06’28”W* |
| 48♀♂ | 90 | Simple | 22m + 14sm + 6st + 6a |
| Ribeiro et al. (2015) |
| abilhoai | Timbó River, Santa Cruz do Timbó, SC, Brazil | 26°30’37.0"S | 50°46’55.0"W |
| 48♀♂ | 90 | Simple | 22m + 14sm + 6st + 6a |
| Ribeiro et al. (2015) |
| aff. dolichopterus Kner, 1854 | Aiapuá Lake, Piagaçu-Purus Sustainable Development Reserve, AM, Brazil | 4°27’26.0"S | 62°11’56.0"W |
| 52♀♂ | 78, 79 | Simple | 16m + 8sm + 2st + 26a ♂, 16m + 9sm + 2st + 25a ♀ | ZZ/ZW | Oliveira et al. (2007) |
| aff. dolichopterus | Aiapuá Lake, Piagaçu-Purus Sustainable Development Reserve, AM, Brazil | 4°27’26.0"S | 62°11’56.0"W |
| 52♀♂ | 78, 79 | Simple | 16m + 8sm + 2st + 26a ♂, 16m + 9sm + 2st + 25a ♀ | ZZ/ZW | Favarato et al. (2016). Reported as Ancistrus sp. Balbina |
| aguaboensis Fisch-Muller, Mazzoni & Weber, 2001 | Ribeirão Bandeirinha, GO, Brazil |
|
| Tocantins-Araguaia | 50♀♂ | 86 | Simple | 16m + 10sm + 4st + 20a |
| Glugoski et al. (2020) |
| aguaboensis | Ribeirão Bandeirinha, GO, Brazil |
|
| Tocantins-Araguaia | 50♀♂ | 86 | Simple | 16m + 10sm + 4st + 20a |
| Schott et al. (2022) |
| cf. dubius Eigenmann & Eigenmann, 1889 | Serra das Araras, Barra do Bugres, MT, Brazil |
|
|
| 44♀♂ |
| Simple | 18m + 10sm + 16st/a | ZZ/ZW | Mariotto et al. (2004) |
| cf. dubius | Coxipó River, Chapada dos Guimarães, MT, Brazil |
|
|
| 42♀♂ | 84 |
| 24m + 10sm + 8st |
| Mariotto et al. (2006) |
| cf. dubius | Pari creek, Cuiabá, MT, Brazil |
|
|
| 42♀♂ | 84 |
| 24m + 10sm + 8st | XX/XY | Mariotto et al. (2006) |
| cf. dubius | Flechas creek, Cáceres, MT, Brazil |
|
|
| 42♀♂ | 84 |
| 24m + 10sm + 8st | XX/XY | Mariotto et al. (2006) |
| cf. dubius | Fundo creek, Poconé, MT, Brazil |
|
|
| 42♀♂ | 84 |
| 24m + 10sm + 8st | XX/XY | Mariotto et al. (2006) |
| cf. dubius | Flechas stream, Brazil | 15°58’07.0"S | 57°19’07.0"W | Paraguai | 42♀♂ | 84 | Simple | 24m + 10sm + 8st | XX/XY | Mariotto et al. (2011) |
| cf. dubius | Fundo stream, Brazil | 16°14’17.0"S | 56°37’31.0"W | Paraguai | 42♀♂ | 84 | Simple | 24m + 10sm + 8st | XX/XY | Mariotto et al. (2011) |
| cf. dubius | Pari stream, Brazil | 15°36’06.0"S | 56°12’19.0"W | Paraguai | 42♀♂ | 84 | Simple | 24m + 10sm + 8st | XX/XY | Mariotto et al. (2011) |
| cf. multispinis (Regan, 1912) | Ribeirão Grande, SP, Brazil |
|
| Paraíba do Sul | 52♀♂ | 84 | Simple | 16m + 10sm + 6st + 20a |
| Glugoski et al. (2020) |
| cf. multispinis | Ribeirão Grande, SP, Brazil |
|
| Paraíba do Sul | 52♀♂ | 84 | Simple | 16m + 10sm + 6st + 20a |
| Schott et al. (2022) |
| cirrhosus (Valenciennes, 1836) | Maracapucú River, Abaetuba, PA, Brazil | 1°45’29.2"S | 48°56’57.0"W | Tocantins-Araguaia | 34♀♂ | 68 | Simple | 20m + 14sm |
| Santos da Silva et al. (2023) |
| cirrhosus | Ilha do Capim, Abaetuba, PA, Brazil | 1°34’02.8"S | 48°51’49.1"W | Tocantins-Araguaia | 34♀♂ | 68 | Simple | 20m + 14sm |
| Santos da Silva et al. (2023). Reported as Ancistrus sp. 2 |
| cirrhosus | Maracapucú River, Abaetuba, PA, Brazil | 1°45’29.2"S | 48°56’57.0"W | Tocantins-Araguaia | 34♀♂ | 68 | Simple | 20m + 14sm |
| Santos da Silva et al. (2022a). Reported as Ancistrus sp. 2 |
| cirrhosus | Ilha do Capim, Abaetuba, PA, Brazil | 1°34’02.8"S | 48°51’49.1"W | Tocantins-Araguaia | 34♀♂ | 68 | Simple | 20m + 14sm |
| Santos da Silva et al. (2022a) |
| claro Knaack, 1999 | Coxipó River, Brazil | 15°21’59.0"S | 55°57’11.0"W | Paraguai | 54♀♂ | 84 | Simple | 14m + 8sm + 8st + 24a |
| Mariotto et al. (2011) |
| claro | Coxipó River, Chapada dos Guimarães, MT, Brazil | 15°21’00.0"S | 55°57’00.0"W | Paraguai | 54♀♂ | 84 | Simple | 14m + 8sm + 8st + 24a |
| Mariotto et al. (2013) |
| clementinae Rendahl, 1937 | Río Palenque (Cantón Pasaje), Ecuador |
|
|
| 52♂, 53♀ | 100, 101 | Simple | 48m/sm + 5a ♀, 48m/sm + 4st/a ♂ | ZZ/ZW1W2 | Nirchio et al. (2023) |
| clementinae | Río La Moquillada (Cantón Las Lajas), Ecuador |
|
|
| 52♂, 53♀ | 100, 101 | Simple | 48m/sm + 5a ♀, 48m/sm + 4st/a ♂ | ZZ/ZW1W2 | Nirchio et al. (2023) |
| cuiabae Knaack, 1999 | Arrombado, Poconé, MT, Brazil | 16°21’21.0"S | 56°27’55.0"W |
| 34♀♂ | 68 | Simple | 20m + 8sm + 6st |
| Mariotto et al. (2009) |
| cuiabae | Arrombado, Poconé, MT, Brazil | 16°21’21.0"S | 56°27’55.0"W |
| 34♀♂ | 67 | Multiple | 19m + 8sm + 6st + 1a |
| Mariotto et al. (2009) |
| cuiabae | Arrombado, Poconé, MT, Brazil | 16°21’21.0"S | 56°27’55.0"W |
| 34♀♂ | 66 | Simple | 18m + 8sm + 6st + 2a |
| Mariotto et al. (2009) |
| cuiabae | Arrombado bay, Brazil | 16°21’21.0"S | 56°27’55.0"W | Paraguai | 34♀♂ | 68 | Simple | 20m + 8sm + 6st |
| Mariotto et al. (2011) |
| dolichopterus Kner, 1854 | Demeni River, Barcelos, AM, Brazil | 0°25’19.0"S | 62°54’42.0"W | Negro | 52♀♂ | 79, 8 | Simple | 12m + 12sm + 4st + 24a ♂, 11m + 12sm + 4st + 25a ♀ | Z1Z1Z2Z2/Z1Z2W1W2 | Oliveira et al. (2008) |
| dolichopterus | Demeni River, Barcelos, AM, Brazil | 0°25’19.0"S | 62°54’42.0"W | Negro | 52♀♂ | 79, 8 | Simple | 12m + 12sm + 4st + 24a ♂, 11m + 12sm + 4st + 25a ♀ | Z1Z1Z2Z2/Z1Z2W1W2 | Favarato et al. (2016) |
| dubius Eigenmann & Eigenmann, 1889 | Barretinho stream, Presidente Figueiredo, AM, Brazil | 1°58’20.0"S | 59°29’48.0"W |
| 38♀, 39♂ | 76, 78 | Simple | 27m + 10sm + 2st ♂, 26m + 10sm + 2st ♀ | XX/XY1Y2 | Oliveira et al. (2008) |
| dubius | Barretinho stream, Presidente Figueiredo, AM, Brazil | 1°58’20.0"S | 59°29’48.0"W |
| 38♀, 39♂ | 76, 78 | Simple | 27m + 10sm + 2st ♂, 26m + 10sm + 2st ♀ | XX/XY1Y2 | Favarato et al. (2016). Reported as Ancistrus sp. Barcelos |
| maximus de Oliveira, Zuanon, Zawadzki & Rapp Py-Daniel, 2015 | Igarapé Macoari, RR, Brazil | 1°10’00.0"N | 61°51’00.0"W |
| 46♀♂ | 81, 82 | Simple | 18m + 11sm + 6st + 11a ♂, 18m + 12sm + 6st + 10a ♀ | XX/XY | Oliveira et al. (2010) |
| maximus | Igarapé Macoari, RR, Brazil | 1°10’00.0"N | 61°51’00.0"W |
| 46♀♂ | 81, 82 | Simple | 18m + 11sm + 6st + 11a ♂, 18m + 12sm + 6st + 10a ♀ | XX/XY | Favarato et al. (2016) |
| multispinis (Regan, 1912) | Itapocu River, SC, Brazil |
|
|
| 52♀♂ |
| Simple | 28m/sm + 24st/a |
| Alves et al. (2003) |
| n. sp. 1 | São Francisco River, AC, Brazil |
|
|
| 38♀♂ |
| Simple | 30m/sm + 8st |
| Alves et al. (2003) |
| n. sp. 1 | Vermelho River, GO , Brazil |
|
|
| 39♂, 40♀ |
| Simple | 33m + 6sm or 34m + 6sm | XX/X0 | Alves et al. (2006) |
| n. sp. 2 | Betari River, SP, Brazil |
|
|
| 52♀♂ |
| Simple | 30m/sm + 20st/a |
| Alves et al. (2003) |
| n. sp. 2 | Garuva River, SC, Brazil |
|
|
| 52♀♂ |
| Simple | 10m + 16sm + 12st + 14a |
| Alves et al. (2006) |
| ranunculus Muller, Rapp Py-Daniel & Zuanon, 1994 | Xingu River, Altamira, PA, Brazil | 3°15’21.0"S | 52°12’45.0"W | Xingu | 48♀♂ | 82 | Simple | 20m + 8sm + 6st + 14a ♂, 19m + 9sm + 6st + 14a ♀ | ZZ/ZW | Oliveira et al. (2007) |
| ranunculus | Xingu River, Altamira, PA, Brazil | 3°15’21.0"S | 52°12’45.0"W | Xingu | 48♀♂ | 82 | Simple | 20m + 8sm + 6st + 14a ♂, 19m + 9sm + 6st + 14a ♀ | ZZ/ZW | Favarato et al. (2016). Reported as Ancistrus sp. Piagaçu |
| sp. | Criminoso stream, MS, Brazil | 18°29’20.0"S | 54°45’14.0"W | Paraguai | 42♀♂ | 84 | Simple | 18m + 16sm + 8st |
| Prizon et al. (2016) |
| sp. | Ivaí River, Brazil |
|
| Ivaí | 50♀♂ | 88 |
| 20m + 12sm + 6st + 12a |
| Barros et al. (2017) |
| sp. | Ivaí River, Brazil |
|
| Ivaí | 50♀♂ | 88 |
| 20m + 12sm + 6st + 12a |
| Schott et al. (2022) |
| sp. 01 | Pipa stream, Serra de São Vicente, MT, Brazil | 14°33’00.0"S | 57°24’00.0"W | Paraguai | 54♀♂ | 84 | Simple | 14m + 8sm + 8st + 24a |
| Mariotto et al. (2013) |
| sp. 03 | Pari stream, Cuiabá, MT, Brazil | 15°36’00.0"S | 56°12’00.0"W | Paraguai | 54♀♂ | 84 | Simple | 14m + 8sm + 8st + 24a |
| Mariotto et al. (2013) |
| sp. 04 | Sepotuba River, Brazil | 14°41’35.0"S | 57°48’14.0"W | Paraguai | 52♀♂ | 82 | Simple | 16m + 8sm + 6st + 22a |
| Mariotto et al. (2011) |
| sp. 04 | São José stream, Sepotuba River, Tangará da Serra, MT, Brazil | 14°41’00.0"S | 57°48’00.0"W | Paraguai | 52♀♂ | 82 | Simple | 16m + 8sm + 6st + 22a |
| Mariotto et al. (2013) |
| sp. 06 | Matrixã River, Brazil | 10°03’07.0"S | 57°36’27.0"W | Amazon | 50♀♂ | 86 | Simple | 18m + 10sm + 8st + 14a |
| Mariotto et al. (2011) |
| sp. 06 | Matrixã River, Nova Monte Verde, MT, Brazil | 10°03’00.0"S | 57°36’00.0"W | Amazon | 50♀♂ | 86 | Simple | 18m + 10sm + 8st + 14a |
| Mariotto et al. (2013) |
| sp. 08 | Currupira River, Brazil | 15°07’59.0"S | 56°49’47.0"W | Paraguai | 44♀♂ | 80 | Simple | 18m + 10sm + 8st + 8a | ZZ/ZW | Mariotto et al. (2011) |
| sp. 1 | Quianduba River, Abaetuba, PA, Brazil | 1°45’18.2"S | 49°00’38.8"W | Tocantins-Araguaia | 38♀♂ | 72 | Simple | 20m + 14sm + 4st | XX/XY | Santos da Silva et al. (2022a) |
| sp. 1 | Quianduba River, Abaetuba, PA, Brazil | 1°45’18.2"S | 49°00’38.8"W | Tocantins-Araguaia | 38♀♂ | 72 | Simple | 20m + 14sm + 4st | XX/XY | Santos da Silva et al. (2023) |
| sp. 1 | Maracapucú River, Abaetuba, PA, Brazil | 1°45’29.2"S | 48°56’57.0"W | Tocantins-Araguaia | 38♀♂ | 72 | Simple | 20m + 14sm + 4st | XX/XY | Santos da Silva et al. (2023) |
| sp. 1 | Ilha do Capim, Abaetuba, PA, Brazil | 1°34’02.8"S | 48°51’49.1"W | Tocantins-Araguaia | 38♀♂ | 72 | Simple | 20m + 14sm + 4st | XX/XY | Santos da Silva et al. (2023) |
| sp. 13 | Salgadinho stream, Brazil | 14°40’14.0"S | 52°21’50.0"W | Tocantins-Araguaia | 40♀♂ | 80 | Simple | 26 m + 10sm + 4st |
| Mariotto et al. (2011) |
| sp. 13 | Salgadinho stream, Nova Xavantina, MT, Brazil | 14°40’00.0"S | 52°21’00.0"W | Tocantins-Araguaia | 40♀♂ | 80 | Simple | 30m + 6sm + 4st |
| Mariotto et al. (2013) |
| sp. Catalão | Catalão Lake, AM, Brazil | 3°10’45.0"S | 59°54’25.0"W | Amazonas | 34♀♂ |
| Simple | 22m + 8sm + 4st | XX/XY | Favarato et al. (2016) |
| sp. Dimona | Fazenda Dimona, Brazil | 2°19’00.0"S | 60°04’00.0"W |
| 52♀♂ | 78 | Simple | 16m + 8sm + 2st + 26a |
| Oliveira et al. (2010) |
| sp. L1 | Mourão River, Brazil |
|
|
| 50♀♂ |
| Simple | 12m + 18sm + 12st + 8a |
| Prizon et al. (2017) |
| sp. L10 | São Francisco Falso River, Brazil |
|
|
| 50♀♂ |
| Simple | 10m + 18sm + 16st + 6a |
| Prizon et al. (2017) |
| sp. L2 | Stream 19, Brazil |
|
|
| 50♀♂ |
| Simple | 12m + 18sm + 12st + 8a ♀, 11m + 18sm + 13st + 8a ♂ | XX/XY | Prizon et al. (2017) |
| sp. L3 | Keller River, Brazil |
|
|
| 50♀♂ |
| Simple | 12m + 18sm + 12st + 8a ♀, 11m + 18sm + 13st + 8a ♂ | XX/XY | Prizon et al. (2017) |
| sp. L6 | São Francisco Verdadeiro River, Brazil |
|
|
| 50♀♂ |
| Simple | 14m + 16sm + 14st + 6a |
| Prizon et al. (2017) |
| sp. L8 | Arroyo San Juan, Misiones, Posada, Argentina |
|
|
| 50♀♂ |
| Simple | 10m + 14sm + 12st + 14a |
| Prizon et al. (2017) |
| sp. L9 | Ocoí River, Brazil |
|
| Paraná | 50♀♂ |
| Simple | 10m + 18sm + 16st + 6a |
| Prizon et al. (2017) |
| sp. Purus | Purus River, AM, Brazil | 4°51’00.0"S | 62°38’00.0"W |
| 34♀♂ | 68 | Simple | 21m + 11sm + 2st ♂, 20m + 12sm + 2st ♀ | XX/XY | Oliveira et al. (2010) |
| sp. Purus | Purus River, AM, Brazil | 4°51’00.0"S | 62°38’00.0"W |
| 34♀♂ | 68 | Simple | 21m + 11sm + 2st ♂, 20m + 12sm + 2st ♀ | XX/XY | Favarato et al. (2016). Reported as Ancistrus sp. Macoari |
| sp. Trombetas | Trombetas River, PA, Brazil | 1°27’00.0"S | 56°21’00.0"W | Trombetas | 38♀♂ | 73 | Simple | 22m + 8sm + 5st + 3a |
| Oliveira et al. (2010) |
| sp. Vermelho | Demeni River, middle Negro River, AM, Brazil | 0°23’00.0"S | 62°50’00.0"W | Negro | 42♀♂ | 78 | Simple | 26m + 6sm + 4st + 6a |
| Oliveira et al. (2010) |
| taunayi Miranda Ribeiro, 1918 | Cascalho stream, Mondaí, SC, Brazil |
|
| Uruguai | 50♀♂ |
| Simple | 22m + 10sm + 10st + 8a | ZZ/ZW | Konerat et al. (2015) |
| tombador Fisch-Muller, Cardoso, da Silva & Bertaco, 2005 | Preto River, Diamantino, MT, Brazil | 14°40’00.0"S | 52°21’00.0"W | Amazon | 50♀♂ | 84 | Simple | 14m + 12sm + 8st + 16a |
| Mariotto et al. (2013) |
Aphanotorulus | emarginatus (Valenciennes, 1840) | Araguaia River, Barra do Garças, MT, Brazil |
|
|
| 52♀♂ |
| Simple | 16m + 30sm + 6st |
| Artoni, Bertollo (2001). Reported as Hypostomus emarginatus |
Araichthys | loro Zawadzki, Bifi & Mariotto, 2016 | Papagaio River, Brazil | 12°79′67”S* | 58°39’02”W* | Amazon | 54♀♂ | 108 | Simple | 36m + 12sm + 6st |
| Mariotto et al. (2019) |
Baryancistrus | aff. niveatus | Xingu River, Altamira, PA, Brazil | 3°12’48.0"S | 52°12’41.7"W | Xingu – Amazon | 52♀♂ | 104 | Simple | 16m + 32sm + 4st |
| Souza et al. (2004) |
| xanthellus Rapp Py-Daniel, Zuanon & de Oliveira, 2011 | Volta Grande do Xingu, Altamira, PA, Brazil | 3°36’31.5"S | 51°34’57.4"W | Xingu-Amazon | 52♀♂ | 104 | Simple | 16m + 28sm + 8st |
| Medeiros et al. (2016) |
| xanthellus | Volta Grande do Xingu, Altamira, PA, Brazil | 3°23’28.2"S | 51°44’29.3"W | Xingu-Amazon | 52♀♂ | 104 | Simple | 16m + 28sm + 8st |
| Medeiros et al. (2016) |
| xanthellus | Volta Grande do Xingu, Altamira, PA, Brazil | 3°22’29.7"S | 51°42’25.0"W | Xingu-Amazon | 52♀♂ | 104 | Simple | 16m + 28sm + 8st |
| Medeiros et al. (2016) |
| xanthellus | Volta Grande do Xingu, Altamira, PA, Brazil | 3°35’38.6"S | 51°49’36.0"W | Xingu-Amazon | 52♀♂ | 104 | Simple | 16m + 28sm + 8st |
| Medeiros et al. (2016) |
Corymbophanes | n. sp. | Chopotó River, Desterro de Melo, MG, Brazil |
|
|
| 54♀♂ |
|
| 20m + 20sm + 14st |
| Alves et al. (2005) |
Hemiancistrus | sp. | Araguaia River, Barra do Garças, MT, Brazil |
|
|
| 52♀♂ |
| Simple | 20m + 20sm + 12st/a |
| Artoni, Bertollo (2001) |
Hypancistrus | cf. debilittera Armbruster, Lujan & Taphorn, 2007 | Uatumã River, AM, Brazil |
|
| Uatumã-Amazon | 52♀♂ | 104 | Multiple | 34m/sm + 18st |
| Santos et al. (2023) |
| cf. debilittera | Uatumã River, AM, Brazil |
|
| Uatumã-Amazon | 52♀♂ | 105 | Multiple | 34m/sm + 18st |
| Silva et al. (2014) |
| L066 | Xingu River, PA, Brazil |
|
| Xingu-Amazon | 52♀♂ | 104 | Multiple | 40m/sm + 12st/a |
| Cardoso et al. (2016) |
| L333 | Xingu River, PA, Brazil |
|
| Xingu-Amazon | 52♀♂ | 104 | Multiple | 40m/sm + 12st/a |
| Cardoso et al. (2016) |
| sp. "Pão" | Xingu River, Belo Monte hydroeletric plant | 3°06’12.8"S | 51°43’53.9"W |
| 52♀♂ | 94 |
| 40m/sm + 12st/a |
| Almeida et al. (2023) |
| sp. "Pão" | Altamira, PA |
|
|
| 52♀♂ | 104 |
| 22m + 18sm + 12st |
| Santos et al. (2023) |
| zebra Isbrücker & Nijssen, 1991 | Xingu River, PA, Brazil |
|
| Xingu-Amazon | 52♀♂ | 104 | Multiple | 34m/sm + 18st |
| Silva et al. (2014) |
| zebra | Xingu River, PA, Brazil |
|
| Xingu-Amazon | 52♀♂ | 104 | Multiple | 40m/sm + 12st/a |
| Cardoso et al. (2016) |
| zebra | Xingu River, Belo Monte hydroeletric plant | 3°06’12.8"S | 51°43’53.9"W |
| 52♀♂ | 94 |
| 40m/sm + 12st/a |
| Almeida et al. (2023) |
| zebra | Altamira, PA |
|
|
| 52♀♂ | 104 |
| 16m + 20sm + 16st |
| Silva et al. (2014) |
Hypostomus | aff. agna (Miranda Ribeiro, 1907) | Cavalo stream, Jaraguá do Sul, SC, Brazil |
|
| Southern Brazil Coast | 74♀♂ |
| Multiple | 8m + 10sm + 32st + 24a |
| Martinez et al. (2011) |
| aff. ancistroides (Ihering, 1911) | São Miguel Arcanjo, SP, Brazil | 23°54’14.3"S | 47°55’46.8"W | Parapanema | 66♀♂ |
| Multiple | 16m + 12sm + 12st + 26a ♀, 17m + 12sm + 12st + 25a ♂ | XX/XY | Rocha-Reis et al. (2018) |
| aff. ancistroides | Keller River, Brazil | 23°38’25.9"S | 51°51’32.8"W |
| 68♀♂ | 119, 118 | Multiple | 16m + 13sm + 22st + 17a ♀, 16m + 12sm + 22st + 18a ♂ | ZZ/ZW | Kamei et al. (2017) |
| aff. ancistroides | Ponta Grossa, PR, Brazil |
|
| Tibagi | 66♀♂ | 104 | Multiple | 12m + 16sm + 10st + 28a |
| Maurutto et al. (2012) |
| aff. cochliodon Kner, 1854 | Esparramo stream, Rondonópolis, MT, Brazil | 16°30’19.6"S | 54°40’31.6"W | Paraguai | 64♀♂ |
| Multiple | 18m + 20sm + 26st/a |
| Becker et al. (2014) |
| aff. cochliodon | Pitaluga stream, Rondonópolis, MT, Brazil | 16°28’38.1"S | 54°32’08.3"W | Paraguai | 64♀♂ |
| Multiple | 18m + 20sm + 26st/a |
| Becker et al. (2014) |
| aff. hermanni (Ihering, 1905) | Keller River, Brazil | 23°38’25.9"S | 51°51’32.8"W |
| 72♀♂ | 124 | Multiple | 12m + 22sm + 18st + 20a |
| Kamei et al. (2017) |
| aff. paulinus (Ihering, 1905) | Piquiri River, PR, Brazil |
|
|
| 74♀♂ |
| Simple | 10m + 12sm + 20st + 32a |
| Bueno et al. (2013) |
| aff. paulinus | Piracicaba River, Piracicaba, SP, Brazil | 22°43’07.0"S | 47°39’19.0"W |
| 76♀♂ | 102 | Simple | 8m + 18sm + 50st/a |
| Rubert et al. (2016) |
| aff. tietensis (Ihering, 1905) | Pirapó River, Brazil | 23°18’15.0"S | 51°53’40.0"W | Paranapanema | 76♀♂ | 114 | Simple | 8m + 6sm + 24st + 38a |
| Paula et al. (2022) |
| aff. tietensis | Do Campo River, Brazil | 24°04’41.0"S | 52°26’12.0"W | Ivaí | 76♀♂ | 114 | Multiple | 8m + 6sm + 24st + 38a |
| Paula et al. (2022) |
| aff. topavae (Gody, 1969) | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 70♀♂ | 102 | Multiple | 18m + 14sm + 38st/a |
| Artoni, Bertollo (1996). Reported as Hypostomus sp. A |
| aff. topavae | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 70♀♂ | 102 | Multiple | 18m + 14sm + 38st/a |
| Lorscheider et al. (2015) |
| aff. unae (Steindachner, 1878) | Contas River, Brazil | 13°51’51.0"S | 40°04’54.0"W | Contas | 76♀♂ | 104 | Simple | 12m + 16sm + 48st/a |
| Bitencourt et al. (2012) |
| aff. unae | Preto do Costa River, Brazil | 13°45’84”S* | 39°56’47”W* | Contas | 76♀♂ | 108 | Simple | 12m + 20sm + 44st/a |
| Bitencourt et al. (2012) |
| aff. unae | Oricó River, Brazil | 14°08’03.0"S | 39°21’30.0"W | Contas | 76♀♂ | 100 | Simple | 10m + 14sm + 52st/a |
| Bitencourt et al. (2012) |
| aff. unae | Preto do Criciúma River, Brazil | 13°55’45.0"S | 39°57’57.0"W | Contas | 76♀♂ | 106 | Simple | 10m + 20sm + 46st/a |
| Bitencourt et al. (2012) |
| affinis (Steindachner, 1877) | Jacuí stream, Brazil | 22°52’00.0"S | 44°51’00.0"W | Paraíba do Sul | 66♀♂ | 106 |
| 14m + 14sm + 12st + 12a |
| Kavalco et al. (2004) |
| affinis | Jacuí stream, Brazil | 22°52’00.0"S | 44°51’00.0"W | Paraíba do Sul | 66♀♂ | 106 | Multiple | 14m + 14sm + 12st + 12a |
| Kavalco et al. (2005) |
| affinis | Jacuí creek, Cunha, SP, Brazil | 23°02’25.9"S | 44°56’02.7"W | Paraíba do Sul | 66♀♂ | 104 | Multiple | 12m + 12sm + 14st + 28a |
| Brandão et al. (2018) |
| affinis | Paraíba do Sul River, Itaocara, RJ, Brazil | 21°39’41.1"S | 42°04’28.3"W | Paraíba do Sul | 66♀♂ | 104 | Multiple | 12m + 12sm + 14st + 28a |
| Brandão et al. (2018) |
| albopunctatus (Regan, 1908) | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 74♀♂ | 104 | Multiple | 10m + 20sm + 44st/a |
| Artoni, Bertollo (1996) |
| albopunctatus | Piquiri River, Brazil |
|
|
| 74♀♂ |
| Multiple | 8m + 14sm + 16st + 36a |
| Bueno et al. (2013) |
| albopunctatus | Piquiri River, Nova Laranjeiras, Brazil | 24°56’54.0"S | 52°35’49.0"W |
| 74♀♂ |
| Simple | 8m + 14sm + 16st + 36a |
| Bueno et al. (2014) |
| albopunctatus | Piracicaba River, Piracicaba, SP, Brazil | 22°43’07.0"S | 47°39’19.0"W |
| 74♀♂ | 104 | Multiple | 10m + 20sm + 44st/a |
| Rubert et al. (2016) |
| albopunctatus | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 74♀♂ | 104 | Multiple | 10m + 20sm + 44st/a |
| Lorscheider et al. (2015) |
| albopunctatus | Piracicaba River, Piracicaba, SP, Brazil | 22°43’07.0"S | 47°39’19.0"W |
| 74♀♂ |
|
| 10 + 18sm + 46st/a |
| Rubert et al. (2022) |
| ancistroides (Ihering, 1911) |
|
|
|
| 68♀♂ | 105M, 106F |
| 10m + 27sm + 31st/a ♂, 10m + 28sm + 30st/a ♀ |
| Michele et al. (1977) |
| ancistroides | Rio Monjolinho, São Carlos, SP, Brazil |
|
|
| 68♀♂ | 102 | Multiple | 16m + 18sm + 34st/a |
| Artoni, Bertollo (1996). Reported as Plecostomus strigaticeps |
| ancistroides | Araquá River, Botucatu, SP, Brazil |
|
|
| 68♀♂ |
| Multiple | 18m + 10sm + 12st + 28a |
| Alves et al. (2006) |
| ancistroides |
|
|
| Paranapanema | 68♀♂ | 104 | Multiple | 10m + 26sm + 32st/a |
| Rubert et al. (2011) |
| ancistroides | Corumbataí River, SP, Brazil |
|
|
| 68♀♂ |
| Multiple | 16m + 4sm + 16st + 32a |
| Alves et al. (2012b) |
| ancistroides | Piquiri River, Formosa do Oeste, PR, Brazil |
|
|
| 68♀♂ |
|
| 14m + 14sm + 8st + 32a |
| Bueno et al. (2012) |
| ancistroides | Dourados stream, Mandaguari, PR, Brazil |
|
| Pirapó | 68♀♂ |
| Multiple | 14m + 12sm + 18st + 24a |
| Endo et al. (2012) |
| ancistroides | Maringá stream, Maringá, PR, Brazil |
|
| Pirapó | 68♀♂ |
| Multiple | 16m + 12sm + 18st + 22a |
| Endo et al. (2012) |
| ancistroides | Ximbaúva stream, Ourizona, PR, Brazil |
|
| Ivaí | 68♀♂ |
| Multiple | 8m + 10sm + 18sm + 32a |
| Endo et al. (2012) |
| ancistroides | Piquiri River, Brazil |
|
|
| 68♀♂ |
| Multiple | 14m + 14sm + 8st + 32a |
| Bueno et al. (2013) |
| ancistroides | Água Boa stream, Mundo Novo, MS, Brazil |
|
|
| 68♀♂ | 116 | Multiple | 14m + 24sm + 10st + 20a |
| Fernandes et al. (2012) |
| ancistroides | Dourado stream, Mundo Novo, MS, Brazil |
|
|
| 68♀♂ | 116 | Multiple | 10m + 22sm + 16st + 20a |
| Fernandes et al. (2012) |
| ancistroides | Dourado stream, Mundo Novo, MS, Brazil |
|
|
| 68♀♂ | 120 | Multiple | 14m + 16sm + 22st + 16a |
| Traldi et al. (2013) |
| ancistroides | Hortelã stream, Botucatu, SP, Brazil | 22°56’28.9"S | 48°35’03.2"W |
| 68♀♂ |
| Multiple | 10m + 20sm + 10st + 28a |
| Pansonato-Alves et al. (2013) |
| ancistroides | Piquiri River, Nova Laranjeiras, Brazil | 24°56’54.0"S | 52°35’49.0"W |
| 68♀♂ |
| Multiple | 14m + 14sm + 8st + 32a |
| Bueno et al. (2014) |
| ancistroides | Monjolinho River, São Carlos, SP, Brazil |
|
|
| 68♀♂ | 102 | Multiple | 16m + 18sm + 34st/a |
| Lorscheider et al. (2015) |
| cf. heraldoi Zawadzki, Weber & Pavanelli, 2008 | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
| Mogi Guaçu | 72♀♂ |
| Simple | 6m + 6sm + 26st + 34a |
| Martinez et al. (2011) |
| cf. plecostomus (Linnaeus, 1758) | Severo stream, Brazil | 9°54’30.8"S | 56°03’33.9"W | Amazon | 68♀♂ | 120/ 121 | Multiple | 14m + 24sm + 14st + 16a ♂, 15m + 24sm + 14st + 15a ♀ | ZZ/ZW | Oliveira et al. (2015) |
| cf. topavae | Carrapato stream, Penápolis, SP, Brazil |
|
| Paraná | 80♀♂ |
| Multiple | 6m + 8sm + 42st + 24a |
| Martinez et al. (2011) |
| cf. wuchereri (Günther, 1864) | Mutum River, Jequié, BA, Brazil | 13°43’18.0"S | 39°51’20.0"W | Contas | 76♀♂ | 104 | Simple | 10m + 18sm + 48st/a |
| Bitencourt et al. (2011b) |
| cf. wuchereri | Una River, Valença, BA, Brazil | 13°21’55.0"S | 39°04’35.0"W | Recôncavo Sul | 76♀♂ | 104 | Simple | 10m + 18sm + 48st/a |
| Bitencourt et al. (2011b) |
| cochliodon Kner, 1854 | Iguaçu River, Brazil |
|
|
| 64♀♂ |
| Simple | 12m + 16sm + 16st + 20a |
| Bueno et al. (2014) |
| cochliodon | Iguaçu River, Foz do Iguaçu, PR, Brazil | 25°38’53.0"S | 54°27’28.0"W |
| 64♀♂ |
| Simple | 12m + 16sm + 16st + 20a |
| Rubert et al. (2016) |
| cochliodon | Piraputanga River, Cáceres, MT, Brazil | 16°03’33.0"S | 57°40’33.0"W | Paraguai | 64♀♂ | 100 | Multiple | 16m + 20sm + 28st/a |
| Rubert et al. (2016) |
| commersoni Valenciennes, 1836 | Iguaçu River, Brazil |
|
|
| 68♀♂ |
| Multiple | 12m + 14sm + 14st + 28a |
| Bueno et al. (2013) |
| commersoni | Lake of Ney Braga hydroeletric plant, Mangueririnha, PR, Brazil |
|
|
| 68♀♂ | 100 | Multiple | 12m + 12sm + 8st + 36a |
| Maurutto et al. (2012) |
| commersoni | Piquiri River, Nova Laranjeiras, Brazil | 24°56’54.0"S | 52°35’49.0"W |
| 68♀♂ |
| Multiple | 12m + 14sm + 14st + 28a |
| Bueno et al. (2014) |
| commersoni | Iguaçu River, Foz do Iguaçu, PR, Brazil | 25°38’53.0"S | 54°27’28.0"W |
| 68♀♂ |
| Multiple | 12m + 14sm + 14st + 28a |
| Bueno et al. (2014) |
| commersoni | Iguaçu River, PR, Brazil | 26°15’01.1"S | 51°06’10.7"W | Paraná | 68♀♂ | 106 | Multiple | 12m + 12sm + 14st + 30a |
| Lorscheider et al. (2018) |
| derbyi (Haseman, 1911) | Iguaçu River, Curitiba, PR, Brazil |
|
|
| 66♀♂ | 82 | Multiple | 6m + 10sm + 20st + 30a |
| Maurutto et al. (2012) |
| derbyi | Iguaçu River, PR, Brazil | 26°15’01.1"S | 51°06’10.7"W | Paraná | 68♀♂ | 102 | Simple | 12m + 12sm + 10st + 34a |
| Lorscheider et al. (2018) |
| faveolus Zawadzki, Birindelli & Lima, 2008 | Taquaralzinho River, MT, Brazil |
|
|
| 64♀♂ |
| Simple | 18m + 8sm + 22st + 16a |
| Bueno et al. (2013) |
| faveolus | Taquaralzinho River, Barra do Garças, MT, Brazil | 15°40’42.0"S | 52°17’52.0"W |
| 64♀♂ |
| Simple | 18m + 8sm + 22st + 16a |
| Bueno et al. (2014) |
| goyazensis (Regan, 1908) | Vermelho River, GO, Brazil |
|
|
| 72♀♂ |
| Simple | 10m + 16sm + 10st + 36a |
| Alves et al. (2006) |
| hermanni (Ihering, 1905) | Piquiri River, PR, Brazil |
|
|
| 72♀♂ |
| Multiple | 10m + 8sm + 32st + 22a |
| Bueno et al. (2013) |
| hermanni | Piquiri River, Nova Laranjeiras, Brazil | 24°56’54.0"S | 52°35’49.0"W |
| 72♀♂ |
| Multiple | 10m + 8sm + 32st + 22a |
| Bueno et al. (2014) |
| hermanni | Piracicaba River, Piracicaba, SP, Brazil | 22°43’07.0"S | 47°39’19.0"W |
| 72♀♂ | 98 | Simple | 8m + 18sm + 46st/a |
| Rubert et al. (2016) |
| hermanni | Piracicaba River, Piracicaba, SP, Brazil | 22°43’07.0"S | 47°39’19.0"W |
| 72♀♂ |
|
| 8m + 18sm + 46st/a |
| Rubert et al. (2022) |
| iheringii (Regan, 1908) | da Lapa stream, Ipeúna, SP, Brazil |
|
|
| 80♀♂ | 132 | Multiple | 8m + 16sm + 28st + 28a |
| Traldi et al. (2012) |
| iheringii | Piracicaba River, Piracicaba, SP, Brazil | 22°43’07.0"S | 47°39’19.0"W |
| 80♀♂ | 102 | Simple | 8m + 14sm + 58st/a |
| Rubert et al. (2016) |
| iheringii | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 76♀♂ | 114 | Simple | 8m + 30sm + 38st/a |
| Artoni, Bertollo (1996). Reported as Hypostomus aff. auroguttatus |
| iheringii | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 76♀♂ | 114 | Simple | 8m + 30sm + 38st/a |
| Lorscheider et al. (2015) |
| iheringii | Piracicaba River, Piracicaba, SP, Brazil | 22°43’07.0"S | 47°39’19.0"W |
| 80♀♂ |
|
| 8m + 14sm + 58st/a |
| Rubert et al. (2022) |
| jaguar Zanata, Sardeiro & Zawadzki, 2013 |
|
|
|
| 76♀♂ |
| Multiple | 10m + 20sm + 46st/a |
| Anjos et al. (2020) |
| macrops (Eigenmann & Eigenmann, 1888) |
|
|
|
| 68♀♂ | 92 |
| 10m + 14sm + 44st/a |
| Michele et al. (1977) |
| mutucae Knaack, 1999 | Claro/Mutuca River, Chapada dos Guimarães, MT, Brazil | 15°20’13.0"S | 55°53’45.0"W | Paraguai | 82♀♂ | 104 | Simple | 4m + 18sm + 60st/a |
| Rubert et al. (2016) |
| mutucae | Claro River, Brazil | 15°20’13.0"S | 55°53’45.0"W |
| 82♀♂ |
| Simple | 4m + 18sm + 60st/a |
| Rubert et al. (2022) |
| myersi (Gosline, 1947) | Iguaçu River, PR, Brazil | 26°15’01.1"S | 51°06’10.7"W | Paraná | 74♀♂ | 114 | Simple | 12m + 16sm + 12st + 34a |
| Lorscheider et al. (2018) |
| nigromaculatus (Schubart, 1964) | Mogi Guaçu River, Cachoeira de Emas, Pirassununga, SP, Brazil |
|
|
| 76♀♂ | 104 | Multiple | 8m + 20sm + 48st/a |
| Rubert et al. (2008) |
| nigromaculatus | Três Bocas stream, Londrina, PR, Brazil |
|
|
| 76♀♂ | 102 | Multiple | 6m + 20sm + 50st/a |
| Rubert et al. (2008) |
| nigromaculatus | dos Apertados stream, Londrina, PR, Brazil |
|
|
| 76♀♂ | 102 | Multiple | 6m + 20sm + 50st/a |
| Rubert et al. (2008) |
| nigromaculatus | Lapa stream, Ipeúna, SP, Brazil |
|
|
| 76♀♂ | 140 | Simple | 12m + 22sm + 30st + 12a |
| Traldi et al. (2013) |
| nigromaculatus | Hortelã stream, Botucatu, SP, Brazil | 22°56’28.9"S | 48°35’03.2"W |
| 76♀♂ |
| Simple | 8m + 18sm + 20st + 30a |
| Pansonato-Alves et al. (2013) |
| nigromaculatus | Pirapitinga River, Caldas Novas, GO, Brazil | 17°43’37.0"S | 48°32’54.0"W |
| 74♀♂ | 104 | Simple | 8m + 22sm + 44st/a |
| Rubert et al. (2016) |
| paulinus (Ihering, 1905) |
|
|
|
| 74♀♂ | 104 |
| 10m + 20sm + 44st/a |
| Michele et al. (1977). Reported as Plecostomus ancistroides |
| paulinus | Três Bocas stream, PR, Brazil |
|
| Paranapanema | 76♀♂ | 98 | Simple | 6m + 16sm + 54st/a |
| Rubert et al. (2011) |
| paulinus | Apertados stream, PR, Brazil |
|
| Paranapanema | 76♀♂ | 98 | Simple | 6m + 16sm + 54st/a |
| Rubert et al. (2011) |
| paulinus |
|
|
|
| 74♀♂ |
| Simple | 10m + 12sm + 20st + 32a |
| Bueno et al. (2014) |
| paulinus | Piracicaba River, Piracicaba, SP, Brazil | 22°43’07.0"S | 47°39’19.0"W |
| 76♀♂ | 98 | Simple | 6m + 16sm + 54st/a |
| Rubert et al. (2016) |
| paulinus | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 72♀♂ | 100 | Multiple | 10m + 18sm + 44st/a |
| Artoni, Bertollo (1996). Reported as Hypostomus sp. C |
| paulinus | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 72♀♂ | 100 | Multiple | 10m + 18sm + 44st/a |
| Lorscheider et al., 2015 |
| paulinus | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 72♀♂ | 106 | Multiple | 14m + 20sm + 38st/a |
| Artoni, Bertollo (1996). Reported as Hypostomus sp. D2 |
| paulinus | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 72♀♂ | 106 | Multiple | 14m + 20sm + 38st/a |
| Lorscheider et al. (2015) |
| paulinus | Piracicaba River, Piracicaba, SP, Brazil | 22°43’07.0"S | 47°39’19.0"W |
| 76♀♂ |
|
| 6m + 16sm + 54st/a |
| Rubert et al. (2022) |
| plecostomus (Linnaeus, 1758) |
|
|
|
| 54♀♂ |
|
|
|
| Hinegardner, Rosen (1972) |
| plecostomus |
|
|
|
| 54♀♂ |
|
| 24m/sm + 12st + 18a |
| Muramoto et al. (1968). Reported as Plecostomus paulinus |
| prope iheringii | Corumbataí River, SP, Brazil |
|
|
| 74♀♂ |
| Multiple | 10m + 14sm + 20st + 30a |
| Alves et al. (2012b) |
| prope paulinus | Corumbataí River, SP, Brazil |
|
|
| 76♀♂ |
| Simple | 6m + 18sm + 12st + 40a |
| Alves et al. (2012b) |
| prope paulinus | Corumbataí River, SP, Brazil |
|
|
| 76♀♂ |
| Simple | 6m + 18sm + 12st + 40a |
| Alves et al. (2012b) |
| prope plecostomus | Orinoco River, Bolivar, Venezuela |
|
|
| 68♀♂ |
| Simple | 12m + 16sm + 12st + 28a |
| Alves et al. (2012b) |
| prope unae | Contas River, Brazil | 13°51’51.0"S | 40°04’54.0"W | Contas | 76♀♂ | 104 | Multiple | 12m + 16sm + 48st/a |
| Bitencourt et al. (2011a) |
| prope unae | Contas River, Brazil | 13°51’51.0"S | 40°04’54.0"W | Contas | 76♀♂ | 108 | Multiple | 12m + 20sm + 44st/a |
| Bitencourt et al. (2011a) |
| prope unae | Oricó River, Brazil | 14°08’03.0"S | 39°21’30.0"W | Contas | 76♀♂ | 100 | Multiple | 10m + 14sm + 52st/a |
| Bitencourt et al. (2011a) |
| prope unae | Preto do Criciúma River, Brazil |
|
| Contas | 76♀♂ | 106 | Multiple | 10m + 20sm + 46st/a |
| Bitencourt et al. (2011a) |
| regani (Ihering, 1905) |
|
|
|
| 72♀♂ |
|
| 10m + 20sm + 42st/a |
| Artoni, Bertollo (1996) |
| regani | Araquá River, Botucatu, SP, Brazil |
|
|
| 72♀♂ |
| Multiple | 12m + 18sm + 26st + 16a |
| Alves et al. (2006) |
| regani | Jacutinga River, Brazil |
|
| Paranapanema | 72♀♂ | 100 | Multiple | 10m + 18sm + 44st/a |
| Rubert et al. (2011) |
| regani | Piumhi River, Piumhi, MG, Brazil | 20°20’31.0"S | 45°59’03.4"W | São Francisco | 72♀♂ | 116 | Simple | 8m + 16sm + 20st + 28a |
| Mendes-Neto et al. (2011) |
| regani | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 72♀♂ |
| Simple | 6m + 6sm + 32st + 28a |
| Martinez et al. (2011) |
| regani | Ivaí River, Floresta, PR, Brazil |
|
| Ivaí | 72♀♂ |
| Multiple | 12m + 14sm + 26st + 20a |
| Endo et al. (2012) |
| regani | Piquiri River, Brazil |
|
|
| 72♀♂ |
| Multiple | 12m + 8sm + 10st + 42a |
| Bueno et al. (2013) |
| regani | Piquiri River, Nova Laranjeiras, Brazil | 24°56’54.0"S | 52°35’49.0"W |
| 72♀♂ |
| Multiple | 12m + 8sm + 10st + 42a |
| Bueno et al. (2014) |
| regani | Mogi Guaçu River, Pirassununga, SP, Brazil | 21°55’37.7"S | 47°22’02.6"W |
| 72♀♂ | 100 | Simple | 10m + 20sm + 42st/a |
| Rubert et al. (2016) |
| regani | Pirapitinga River, Caldas Novas, GO, Brazil | 17°43’37.0"S | 48°32’54.0"W |
| 72♀♂ | 106 | SImple | 12m + 22sm + 38st/a |
| Rubert et al. (2016) |
| regani | Onça stream, Coxim, MS, Brazil | 18°32’18.0"S | 54°33’43.0"W | Paraguai | 72♀♂ |
| Simple | 12m + 14sm + 18st + 28a |
| Ferreira et al. (2019) |
| regani | Onça stream, Coxim, MS, Brazil | 18°32’18.0"S | 54°33’43.0"W | Paraguai | 72♀♂ |
| Simple | 13m + 14sm + 17st + 18a |
| Ferreira et al. (2019) |
| soniae Hollanda Carvalho & Weber, 2005 | Alta Floresta, MT, Brazil | 9°54’30.8"S | 56°03’33.9"W | Teles Pires | 64♀♂ | 112 | Multiple | 12m + 22sm + 14st + 16a | XX/XY | Oliveira et al. (2019) |
| soniae | Alta Floresta, MT, Brazil | 9°53’50.5"S | 56°03’39.5"W | Teles Pires | 64♀♂ | 112 | Multiple | 12m + 22sm + 14st + 16a | XX/XY | Oliveira et al. (2019) |
| soniae | Alta Floresta, MT, Brazil | 9°53’30.5"S | 56°04’18.8"W | Teles Pires | 64♀♂ | 112 | Multiple | 12m + 22sm + 14st + 16a | XX/XY | Oliveira et al. (2019) |
| sp. | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 72♀♂ | 108 | Multiple | 10m + 26sm + 36st/a |
| Artoni, Bertollo (1996). Reported as Hypostomus sp. D1 |
| sp. | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 72♀♂ | 108 | Multiple | 10m + 26sm + 36st/a |
| Lorscheider et al. (2015) |
| sp. | Fundo stream, Barra do Garças, MT, Brazil |
|
| Tocantins-Araguaia | 64♀♂ |
| Simple | 14m + 24sm + 26st/a ♂, 15m + 24sm + 25st/a ♀ | ZZ/ZW | Artoni et al. (1998) |
| sp. | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
| Mogi Guaçu | 68♀♂ |
| Multiple | 6m + 6sm + 32st + 24a |
| Martinez et al. (2011) |
| sp. | Esparramo stream, Rondonópolis, MT, Brazil | 16°30’00.0"S | 54°40’00.0"W | Paraguay | 74♀♂ |
| Multiple | 12m + 20sm + 42st/a |
| Becker et al. (2014) |
| sp. 2 Rio Perdido | Perdido River, Planalto da Bodoquena, MS, Brazil | 21°17’09.0"S | 56°41’46.0"W |
| 84♀♂ | 106 | Simple | 6m + 16sm + 62st/a |
| Cereali et al. (2008) |
| sp. 3 Córrego Salobrinha | Salobra River, Planalto da Bodoquena, MS, Brazil | 20°41’34.0"S | 56°44’25.0"W |
| 82♀♂ | 100-102 |
| 6m + 12sm + 64st/a + 1-2aBs |
| Cereali et al. (2008) |
| sp. 3 Córrego Salobrinha | Salobrinha stream, Planalto da Bodoquena, MS, Brazil | 20°41’07.0"S | 56°46’44.0"W |
| 82♀♂ | 100-102 |
| 6m + 12sm + 64st/a + 1-2aBs |
| Cereali et al. (2008) |
| sp. B | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 72♀♂ |
| Simple | 12m + 18sm + 42st/a |
| Artoni et al. (1999a) |
| sp. E | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 80♀♂ |
| Multiple | 8m + 16sm + 56st/a |
| Artoni et al. (1999a) |
| sp. F | São Francisco River, Brazil |
|
|
| 75, 76♀♂ |
|
| 10m + 16sm + 50st/a or 10m + 17sm + 48st/a |
| Artoni et al. (1999a) |
| sp. Xingu-1 | Xingu River, Altamira, PA, Brazil | 3°12’48.0"S | 52°12’41.7"W | Xingu – Amazon | 64♀♂ |
| Simple | 32m/sm + 32st/a |
| Milhomem et al. (2010) |
| sp. Xingu-2 | Xingu River, Altamira, PA, Brazil | 3°12’48.0"S | 52°12’41.7"W | Xingu – Amazon | 66♀♂ |
| Simple | 32m/sm + 34st/a |
| Milhomem et al. (2010) |
| sp. Xingu-3 | Xingu River, Altamira, PA, Brazil | 3°12’48.0"S | 52°12’41.7"W | Xingu – Amazon | 65♀♂ |
| Simple | 38m/sm + 26st/a + 1B |
| Milhomem et al. (2010) |
| spiniger (Hensel, 1870) | Forquetinha River, Forquetinha, RS, Brazil | 29°21’43.5’’S | 52° 07’39.6’’W |
| 66♀♂ |
| Multiple | 10m + 16sm + 14st + 26a |
| Takagui et al. (2023) |
| spiniger | Quadros Lagoon, RS, Brazil | 29°21’43.5’’S | 52° 07’39.6’’W |
| 66♀♂ |
| Multiple | 10m + 16sm + 14st + 26a |
| Takagui et al. (2023) |
| spiniger | Forquetinha River, Forquetinha, RS, Brazil | 29°21’43.5"S | 52°07’39.6"W |
| 66♀♂ | 92 | Multiple | 10m + 16sm + 40st/a |
| Rubert et al. (2016). Reported as Hypostomus commersoni, corrected in Takagui et al. (2023) |
| strigaticeps (Regan, 1908) |
|
|
|
| 74♀♂ | 86 |
| 8m + 4sm + 62st/a |
| Michele et al. (1977). Reported as Plecostomus macrops |
| strigaticeps |
|
|
| Paranapanema | 72♀♂ | 98 | Multiple | 10m + 16sm + 46st/a |
| Rubert et al. (2011) |
| strigaticeps | Corumbataí River, SP, Brazil |
|
|
| 74♀♂ |
| Multiple | 10m + 14sm + 14st + 36a |
| Alves et al. (2012b) |
| strigaticeps | Ivaí River, Floresta, PR, Brazil |
|
|
| 72♀♂ |
| Multiple | 10m + 14sm + 18st + 30a |
| Endo et al. (2012) |
| strigaticeps | Piquiri River, Brazil |
|
|
| 72♀♂ |
| Multiple | 12m + 12sm + 18st + 30a |
| Bueno et al. (2013) |
| strigaticeps | Água Boa stream, Mundo Novo, MS, Brazil |
|
|
| 72♀♂ | 122 | Multiple | 12m + 18sm + 20st + 22a |
| Fernandes et al. (2012) |
| strigaticeps | Hortelã stream, Botucatu, SP, Brazil | 22°56’28.9"S | 48°35’03.2"W |
| 72♀♂ |
| Multiple | 10m + 18sm + 18st + 26a |
| Pansonato-Alves et al. (2013) |
| strigaticeps | Piquiri River, Nova Laranjeiras, Brazil | 24°56’54.0"S | 52°35’49.0"W |
| 72♀♂ |
| Multiple | 12m + 12sm + 18st + 30a |
| Bueno et al. (2014) |
| strigaticeps | Atlântico stream, PR, Brazil | 23°18’13.0"S | 52°01’51.0"W | Paraná | 72♀♂ |
| Multiple | 12m + 12sm + 18st + 30a |
| Baumgärtner et al. (2014) |
| strigaticeps | Piquiri River, PR, Brazil | 24°56’54.0"S | 52°35’49.0"W | Paraná | 72♀♂ |
| Multiple | 12m + 12sm + 18st + 30a |
| Baumgärtner et al. (2014) |
| strigaticeps | Paraná River, PR, Brazil | 24°48’43.0"S | 54°19’16.0"W | Paraná | 72♀♂ |
| Multiple | 12m + 12sm + 18st + 30a |
| Baumgärtner et al. (2014) |
| strigaticeps | Iguassu River, PR, Brazil | 25°39’02.0"S | 54°27’25.0"W | Paraná | 72♀♂ |
| Multiple | 12m + 12sm + 18st + 30a |
| Baumgärtner et al. (2014) |
| strigaticeps | Mogi Guaçu River, Pirassununga, SP, Brazil | 21°55’37.7"S | 47°22’02.6"W |
| 72♀♂ | 98 | Simple | 10m + 16sm + 46st/a |
| Rubert et al. (2016) |
| strigaticeps | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 72♀♂ | 102 | Simple | 12m + 18sm + 42st/a |
| Lorscheider et al. (2015) |
| strigaticeps | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 72♀♂ | 102 | Simple | 12m + 18sm + 42st/a |
| Artoni, Bertollo (1996). Reported as Hypostomus sp. B |
| tapijara Oyakawa, Akama & Zanata, 2005 | Ribeira de Iguape River, Registro, SP, Brazil |
|
|
| 66♀♂ | 118 | Multiple | 14m + 24sm + 14st + 14a |
| Traldi et al. (2013) |
| tietensis (Ihering, 1905) | Piraí River, Brazil | 23°22’22.0"S | 47°22’13.0"W | Paraná | 72♀♂ | 108 | Simple | 8m + 8sm + 20st + 36a |
| Anjos et al. (2020) |
| tietensis | Piraí River, Brazil | 23°22’22.0"S | 47°22’13.0"W | Paraná | 72♀♂ | 109 | Simple | 8m + 8sm + 20st + 36a |
| Paula et al. (2022) |
| topavae (Godoy, 1969) | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 80♀♂ | 104 | Multiple | 8m + 16sm + 56st/a |
| Artoni, Bertollo (1996). Reported as Hypostomus sp. E |
| topavae | Mogi Guaçu River, Pirassununga, SP, Brazil |
|
|
| 80♀♂ | 104 | Multiple | 8m + 16sm + 56st/a |
| Lorscheider et al. (2015) |
| topavae | Piquiri River, Nova Laranjeiras, PR, Brazil |
|
|
| 80♀♂ |
|
| 14m + 10sm + 26st + 30a |
| Bueno et al. (2012) |
| topavae | Piquiri River, PR, Brazil |
|
|
| 80♀♂ |
| Simple | 14m + 10sm + 26st + 30a |
| Bueno et al. (2013) |
| topavae | Piquiri River, Nova Laranjeiras, PR, Brazil | 24°56’54.0"S | 52°35’49.0"W | Paraná | 80♀♂ |
| Simple | 14m + 10sm + 26st + 30a |
| Bueno et al. (2014) |
| topavae | Keller River, Brazil | 23°38’25.9"S | 51°51’32.8"W |
| 80♀♂ | 142 | Multiple | 14m + 30sm + 18st + 18a |
| Kamei et al. (2017) |
| unae (Steindachner, 1878) |
|
|
|
| 76♀♂ |
|
| 10m + 20sm + 46st/a |
| Anjos et al. (2020) |
Lasiancistrus | schomburgkii (Günther, 1864) | Massangana River, Brazil | 9°80′48″S* | 63°08′90″W* | Amazon | 54♀♂ | 108 | Simple | 28m + 16sm + 10st |
| Mariotto et al. (2019) |
| sp. | Cachoeira River, Brazil | 14°64′66″S* | 52°35′50″W* | Tocantins-Araguaia | 54♀♂ | 108 | Simple | 28m + 16sm + 10st |
| Mariotto et al. (2019) |
Megalancistrus | sp. | Cuiabá River, Brazil | 15°62′90″S* | 56°08′70″W* | Paraguai | 52♀♂ | 104 | Simple | 28m + 16sm + 8st |
| Mariotto et al. (2019) |
| parananus (Peters, 1881) | Piquiri River, Nova Laranjeiras, PR, Brazil |
|
|
| 52♀♂ |
| Simple | 18m + 24sm + 10st |
| Bueno et al. (2018) |
Panaqolus | sp. | Camarapi River, Portel, PA, Brazil |
|
|
| 52♀♂ |
| Simple | 24m + 18sm + 10st/a |
| Ayres-Alves et al. (2017) |
| tankei Cramer & Sousa, 2016 | Xingu River, Brazil |
|
|
| 52♀♂ | 104 | Simple | 6m + 38sm + 8st |
| Ferreira et al. (2021) |
Panaque | armbrusteri Lujan, Hidalgo & Stewart, 2010 | Xingu River, Altamira, PA, Brazil |
|
|
| 52♀♂ |
| Simple | 26m + 20sm + 6st/a |
| Ayres-Alves et al. (2017) |
| armbrusteri | Gorgulho da Rita, Altamira, PA, Brazil |
|
|
| 52♀♂ |
| Simple | 26m + 20sm + 6st/a |
| Ayres-Alves et al. (2017) |
| cf. nigrolineatus | Araguaia River, Barra do Garças, MT, Brazil |
|
|
| 52♀♂ |
| Simple | 26m + 20sm + 6st/a |
| Artoni, Bertollo (2001) |
Peckoltia | cavatica Armbruster & Werneke, 2005 | PA, Brazil |
|
|
| 52♀♂ |
| Simple | 38m/sm + 14st |
| Pety et al. (2018) |
| multispinis (Holly, 1929) | PA, Brazil |
|
|
| 52♀♂ |
| Simple | 28m/sm + 24st |
| Pety et al. (2018) |
| oligospila (Günther, 1864) | PA, Brazil |
|
|
| 52♀♂ |
| Multiple | 38m/sm + 14st |
| Pety et al. (2018) |
| sabaji Armbruster, 2003 | PA, Brazil |
|
|
| 52♀♂ |
| Multiple | 38m/sm + 14st |
| Pety et al. (2018) |
| sp. 1 Jari river | Jari River, Monte Dourado, PA, Brazil | 03°18’14.9"N | 52°03’29.3"W |
| 52♀♂ | 102 | Multiple | 44m/sm + 6st + 2a + 1B |
| Souza et al. (2009) |
| sp. 2 Jari river | Jari River, Monte Dourado, PA, Brazil | 03°18’14.9"N | 52°03’29.3"W |
| 52♀♂ | 102 |
| 32m/sm + 18st + 2a |
| Souza et al. (2009) |
| sp. 3 Jarumã | Abaetuba, PA, Brazil | 1°42’41.9"S | 48°51’45.9"W |
| 52♀♂ |
| Simple | 46m/sm + 6st |
| Silva et al. (2021) |
| sp. 4 Caripetuba | Abaetuba, PA, Brazil | 1°37’23.5"S | 48°55’33.0"W |
| 52♀♂ |
| Multiple | 40m/sm + 12st |
| Silva et al. (2021) |
| vittata (Steindachner, 1881) | Xingu River, Altamira, PA, Brazil | 03°12’4"N | 52°12’41.7"W |
| 52♀♂ | 102 | Simple | 16m + 20sm + 14st + 2a |
| Souza et al. (2009) |
| vittata | PA, Brazil |
|
|
| 52♀♂ |
| Simple | 32m/sm + 18st + 2a |
| Pety et al. (2018) |
Pseudacanthicus | leopardus (Fowler, 1914) | Xingu River, Brazil |
|
| Xingu – Amazon | 52♀♂ | 104 | Simple | 18m + 34sm |
| Santos da Silva et al. (2022b) |
| sp. | Xingu River, Brazil |
|
| Xingu – Amazon | 52♀♂ | 104 | Simple | 18m + 34sm |
| Santos da Silva et al. (2022b) |
| spinosus (Castelnau, 1855) | Tocantins River, Brazil |
|
| Tocantins-Araguaia | 52♀♂ | 104 | Simple | 18m + 34sm |
| Santos da Silva et al. (2022b) |
Pterygoplichthys | ambrosettii (Holmberg, 1893) | Preto River, Mirassolândia, SP, Brazil |
|
|
| 52♀♂ |
| Simple | 16m + 24sm + 8st + 4a |
| Artoni et al. (1999b). Reported as Liposarcus anisitsi |
| ambrosettii | Tietê River, Botucatu, SP, Brazil |
|
|
| 52♀♂ |
| Simple | 28m + 12sm + 8st + 4a |
| Alves et al. (2006). Reported as Liposarcus anisitsi |
| ambrosettii | Miranda River, MS, Brazil |
|
|
| 52♀♂ |
| Simple | 8m + 14sm + 14st + 16a |
| Alves et al. (2006). Reported as Liposarcus anisitsi |
| ambrosettii | Agua Boa stream, Brazil | 23°50’16.7"S | 54°20’55.5"W |
| 52♀♂ | 100 | Simple | 14m + 26sm + 8st + 4a |
| Fernandes et al. (2015). Reported as Pterygoplichthys anisitsi |
| ambrosettii | Paraná River, Guaíra, PR, Brazil |
|
|
| 52♀♂ |
| Simple | 16m + 24sm + 8st + 4a |
| Bueno et al., 2018 |
| chrysostiktos (Birindelli, Zanata & Lima, 2007) |
|
|
|
| 52♀♂ |
| Simple | 22m + 20sm + 10st |
| Anjos et al. (2020). Reported as Hypostomus chrysostiktos |
| gibbiceps (Kner, 1854) | Orinoco River, Venezuela |
|
|
| 52♀♂ |
| Simple | 20m + 24sm + 8st |
| Alves et al. (2006). Reported as Glyptoperichthys gibbiceps |
| joselimaianus (Weber, 1991) | Quatro Bocas Lake, Araguaiana, MT, Brazil | 15°23’20.1"S | 51°42’45.9"W |
| 52♀♂ |
| Simple | 28m + 16sm + 8st/a |
| Oliveira et al. (2006) |
| multiradiatus (Hancock, 1828) | Orinoco River, Venezuela |
|
|
| 52♀♂ |
| Simple | 22m + 18sm + 12st |
| Alves et al. (2006). Reported as Liposarcus multiradiatus |
| multiradiatus | Orinoco River, Venezuela |
|
|
| 52♀♂ |
| Simple | 22m + 18sm + 12st |
| Alves et al. (2012a). Reported as Liposarcus multiradiatus |
| pardalis (Castelnau, 1855) | Porto dos Milagres, Amazonas River, Santarém, PA, Brazil |
|
|
| 52♀♂ |
| Simple | 18m + 18sm + 8st + 8a |
| Guimarães et al. (2023) |
| pardalis | Arapixuna, Amazonas River, Santarém, PA, Brazil |
|
|
| 52♀♂ |
| Simple | 18m + 18sm + 8st + 8a |
| Guimarães et al. (2023) |
| pardalis | Grande Lake, Amazonas River, Santarém, PA, Brazil |
|
|
| 52♀♂ |
| Simple | 18m + 18sm + 8st + 8a |
| Guimarães et al. (2023) |
| pardalis | Pacoval Lake, Amazonas River, Santarém, PA, Brazil |
|
|
| 52♀♂ |
| Simple | 18m + 18sm + 8st + 8a |
| Guimarães et al. (2023) |
Scobinancistrus | aureatus Burgess, 1994 | Xingu River, Brazil |
|
| Xingu-Amazon | 52♀♂ |
| Simple | 22m + 20sm + 10st |
| Cardoso et al. (2013) |
| aureatus | Xingu River, Brazil |
|
| Xingu-Amazon | 52♀♂ |
| Simple | 22m + 20sm + 10st |
| Ayres-Alves et al. (2017) |
| aureatus | Xingu River, Belo Monte hydroeletric plant, Brazil | 3°06’12.8"S | 51°43’53.9"W |
| 52♀♂ |
|
| 24m + 18sm + 10st |
| Almeida et al. (2023) |
| aureatus | Xingu River, Gorgulho da Rita, Brazil | 3°20’06.2"S | 52°10’32.9"W |
| 52♀♂ |
|
| 24m + 18sm + 10st |
| Almeida et al. (2023) |
| pariolispos Isbrücker & Nijssen, 1989 | Xingu River, Brazil |
|
| Xingu-Amazon | 52♀♂ |
| Simple | 24m + 18sm + 10st |
| Cardoso et al. (2013) |
| pariolispos | Xingu River, Brazil |
|
| Xingu-Amazon | 52♀♂ |
| Simple | 24m + 18sm + 10st |
| Ayres-Alves et al. (2017) |
| pariolispos | Xingu River, Belo Monte hydroeletric plant, Brazil | 3°06’12.8"S | 51°43’53.9"W |
| 52♀♂ |
|
| 22m + 20sm + 10st |
| Almeida et al. (2023) |
Transancistrus | santarosensis (Tan & Armbruster, 2012) | Río Dos Bocas, Río Palenque, Ecuador |
|
|
| 54♀♂ | 106 | Multiple | 25m + 27sm + 2a ♀, 26m + 26sm + 2a ♂ | ZZ/ZW | Tursellino et al. (2023) |
Delturinae | |||||||||||
Hemipsilichthys | sp. | Paraitinga River, Brazil | 22°52’22.5"S | 44°51’00.4"W | Paraíba do Sul | 96♀♂ | 120 |
| 16m + 8sm + 72a |
| Kavalco et al. (2004). Reported as Upsilodus sp. |
| sp. | Paraitinga River, Brazil | 22°52’22.5"S | 44°51’00.4"W | Paraíba do Sul | 96♀♂ | 120 | Simple | 16m + 8sm + 72a |
| Kavalco et al. (2005) |
| n. sp. | Patos River, Lapa, PR, Brazil |
|
|
| 54♀♂ |
|
| 20m + 30sm + 4st |
| Alves et al. (2005) |
Rhinelepinae | |||||||||||
Pogonopoma | wertheimeri (Steindachner, 1867) | Mucurí River, Taquarinha, BA, Brazil |
|
|
| 54♀♂ |
| Multiple | 20m + 30sm + 4st |
| Artoni, Bertollo (2001) |
Rhinelepis | aspera Spix & Agassiz, 1829 | Paraná River, Guaíra, PR, Brazil |
|
|
| 54♀♂ |
| Simple | 20m + 26sm + 8st |
| Artoni, Bertollo (2001). Reported as Rhinelepis aspera |
| aspera | Paraná River, Porto Rico, PR, Brazil |
|
|
| 54♀♂ |
| Simple | 24m + 18sm + 12st |
| Endo et al. (2012) |
| aspera | Paraná River, Santa Helena, PR, Brazil |
|
|
| 54♀♂ |
| Simple | 20m + 26sm + 8st |
| Bueno et al. (2018) |
Discussion
The origin of the chromosomal diversity of Loricariidae is attributed to both ecological and molecular factors. Small and isolated populations, in addition to the low vagility, are the main ecological characteristics that have allowed the fixation of chromosomal rearrangements in Loricariidae, as suggested for Farlowella (Marajó et al., 2018), Harttia (Sassi et al., 2023), and Rineloricaria (Rosa et al., 2012). The diploid number 2n = 54 is often considered the ancestral diploid number for the family, since it is observed in most Loricariidae subfamilies and present in other Siluriformes (Artoni, Bertollo, 2001). However, there is no consensus on the matter particularly considering the new karyotypic description of basal taxa within subfamilies. Takagui et al. (2020) in a review of Loricariinae karyotypes argue that although predominant, 2n = 54 should not be considered the basal diploid number for the family because multiple divergences in the microstructure of karyotypes within the same 2n are recurrently seen throughout the family. Notably, the 2n range in Loricarioidei suggest that other numbers rather than 2n = 54 can be considered the plesiomorphic ones, as Astroblepidae presents 2n = 52–54, Scoloplacidae 2n = 50, Callichthyidae 2n = 40–134, and Trichomycteridae 2n = 32–62 (reviewed by Conde-Saldaña et al., 2018). Beyond that, the Diplomystidae (Siluroidei) presents 2n = 56 chromosomes (Campos et al., 1997; Oliveira, Gosztonyi, 2000), which might also suggest that as the ancestral 2n.
We plotted the 2n range per subfamily into the main molecular phylogenetic reconstructions of Loricariidae (Fig. 3) and 2n = 54 is the most widespread, being conserved in Rhinelepinae and present in all other subfamilies. Regardless matter whether 2n = 54 or 2n = 56 is the ancestral diploid number, it is noteworthy that chromosomal evolution in Loricariidae is complex. Considering the lower number of karyotyped species when compared to the valid richness, 234 spp. with cytogenetic data against 1,051 valid species (Fricke et al., 2023), it is difficult to establish the evolutionary pathways that led to the observed variability. Notably, while the stability is restricted to 2n in some genera, with high divergence in karyotype structure, as the 2n = 58 in Farlowella, 2n = 54 in Corumbataia, and 2n = 52 in Pterygoplichthys, stable 2n and karyotype is also observed, as the 2n = 52 (26m+20sm+6st/a) in two species of Panaque. Our compilation reveals that processes of ascending and descending dysploidy (i.e., the increase or decrease of 2n while preserving the genomic content) were frequent in most subfamilies but Rhinelepinae, which may exhibit the constant 2n = 54 as a symplesiomorphic trait.
FIGURE 3| Phylogenetic relationships of Loricariidae with cytogenetic data herein compiled in red. Molecular phylogenies modified from: A. Roxo et al. (2014); B. Lujan et al. (2015); C. Pereira, Reis (2017); D. Roxo et al. (2019).
Despite the 2n conservation in Rhinelepinae, variations in the karyotype structure are observed within and between species. Populations of Rhinelepis aspera Spix & Agassiz, 1829 from the same river differ in the karyotype formula (Artoni, Bertollo, 2001; Endo et al., 2012), suggesting that pericentric inversions played an important role in the chromosomal evolution of the species. Such mechanism is not restricted to Rhinelepinae but also observed in other loricariids, such as Ancistrus (Mariotto et al., 2009), Loricariichthys (Takagui et al., 2014), and Rineloricaria (Rosa et al., 2012; Primo et al., 2018). In addition, the multiple Ag-NOR observed in Pogonopoma wertheimeri (Steindachner, 1867) (Artoni, Bertollo, 2001) when compared to the simple distribution in other Rhinelepinae species suggest that other mechanisms are also important for the chromosomal evolution in the group. Notably, numeric and structural polymorphisms are frequently observed in loricariids, for example in the multiple karyomorphs of Rineloricaria pentamaculata Langeani & de Araujo, 1994 (Glugoski et al., 2023), and the presence of B chromosomes in Harttia longipinna Langeani, Oyakawa & Montoya-Burgos, 2001(Blanco et al., 2012). This chromosomal diversity was probably generated by a combination of rearrangements that include Robertsonian fusions and fissions, paracentric and pericentric inversions, and translocations (Artoni, Bertollo, 2001; Kavalco et al., 2004; Ziemniczak et al., 2012).
Although rare in most fish species, being observed in only about 5% of Teleostei species (Arai, 2011; Sember et al., 2021), our compilation shows that Loricariidae species carry seven out of the nine known sex chromosome systems observed among fishes. Ancistrus was demonstrated to harbor the largest diversity of sex chromosomes with six of the seven recognized systems for the family distributed in 18 species, corresponding to about 23% of the valid species (Neuhaus et al., 2023). The simple XX/XY was the most predominant in the genus, recorded in A. maximus de Oliveira, Zuanon, Zawadzki & Rapp Py-Daniel, 2015 (Oliveira et al., 2010; Favarato et al., 2016), Ancistrus cf. dubius (Mariotto et al., 2011), Ancistrus sp. 1 Quianduba River (Silva et al., 2022, 2023), Ancistrus sp. 1 Maracapucú River (Santos da Silva et al., 2023), Ancistrus sp. 1 Ilha do Capim (Santos da Silva et al., 2023), Ancistrus sp. Catalão (Favarato et al., 2016), Ancistrus sp. L2 (Prizon et al., 2017), Ancistrus sp. L3 (Prizon et al., 2017), and Ancistrus sp. Purus (Oliveira et al., 2010; Favarato et al., 2016). Additionally, two multiple sex chromosome systems that were not observed in any other Loricariidaeare recorded in Ancistrus: ZZ/ZW1W2 in A. clementinae Rendahl, 1937 (Nirchio et al., 2023), and Z1Z1Z2Z2/Z1Z2W1W2 (Oliveira et al., 2008; Favarato et al., 2016). On other hand, Harttia harbor the highest number of multiple sex chromosome systems when compared to the number of valid species, with six occurrences representing about 25% of valid species (compiled in Sassi et al., 2021): XX/XY1Y2 in H. carvalhoi Miranda Ribeiro, 1939, H. intermontana Oliveira & Oyakawa, 2019, and Harttia sp. 1 (Centofante et al., 2006; Deon et al., 2020); X1X1X2X2/X1X2Y in H. duriventris Rapp Py-Daniel & Oliveira, 2001, H. punctata Rapp Py-Daniel & Oliveira, 2001, and H. villasboas Oyakawa, Fichberg & Rapp Py-Daniel, 2018 (Blanco et al., 2014; Sassi et al., 2020), in addition to putative simple XX/XY in H. rondoni Oyakawa, Fichberg & Rapp Py-Daniel, 2018 and H. torrenticola Oyakawa, 1993 (Deon et al., 2020; Sassi et al., 2020). The Loricariidae diversity of sex chromosomes was originated by rearrangements that include translocations (Blanco et al., 2014), centric fissions (Sassi et al., 2023); centric fusions (Centofante et al., 2006), and pericentric inversions (Artoni et al., 1998), including the combination of distinct rearrangements especially in the origin of multiple sex chromosome systems (Oliveira et al., 2008; Deon et al., 2022). Sex chromosomes in Loricariidae seems to have independent origins, but further research is required to explore the genomic content of those sex chromosomes and its origin. Indeed, there is a recognized lack of information regarding the effects of environmental cues and molecular/gene mechanisms in sex determination of Neotropical fishes (Fernandino, Hattori, 2019).
Most Loricariidae species present a single 18S rDNA/Ag-NOR site, which is also considered a plesiomorphic character in the group (Artoni, Bertollo, 2001; Kavalco et al., 2004), and the standard distribution in most vertebrates (Sochorová et al., 2018). Notably, such region in loricariids is involved in several chromosomal rearrangements, including the origin and differentiation of sex chromosomes, being considered evolutionary breakpoint regions in Ancistrus, Harttia, and Rineloricaria (Glugoski et al., 2018; Deon et al., 2022). Size heteromorphism in the Ag-NOR site is also common, probably because of unequal crossing-over between homologs (Takagui et al., 2020). According to our review, some genus conserved the simple Ag-NOR locus in all analyzed species to date (here included only those genera with more than one species karyotyped), namely as Baryancistrus, Corumbataia, Farlowella, Harttia, Hisonotus, Lasiancistrus, Loricaria, Loricariichthys, Neoplecostomus, Panaqolus, Panaque, Pareiorhina, Pseudacanthicus, Pterygoplichthys, and Scobinancistrus. On other hand, the multiple distribution of Ag-NOR seems to be conserved only in Hypancistrus, while other genus as Ancistrus, Hypostomus, and Rineloricaria present both simple and multiple distributions on chromosomes.
Although distributed throughout the Neotropical region, there is a predominance of cytogenetic studies in Loricariidae species from Brazil (Fig. 2). Few studies were conducted in other countries that includes Argentina, Ecuador, Paraguay, and Venezuela. Notably, those in Argentina and Paraguay were mostly restricted to the frontier region with Brazil. When accounting the Brazilian territory, is also notable that some regions are poorly represented in cytogenetic studies, especially the northeast in which at least nine states have not been included in the cytogenetic samplings. In addition, the Guianas Shield and Western Amazon regions are largely recognized as neglected regions in biogeographical and evolutionary studies (Cassemiro et al., 2023), also with little or absent cytogenetic information for Loricariidae. Despite the regional sampling gap problem, Loricariidae diversity is still insufficiently represented by cytogenetic studies. Our compilation recorded 234 species assessed by cytogenetic studies, that in comparison to the 1,051 valid species (Fricke et al., 2023), represents 22.26% of the family species richness. The diversity of genus assessed by cytogenetic studies was the highest in Hypoptopomatinae (42.1%), followed by Rhinelepinae (28.5%), Hypostominae (27.2%), Loricariinae (24.4%), Delturinae (20%), and Lithogeninae (0%). Besides Lithogeninae that do not have any species karyotyped to date, the subfamily Delturinae has only one genus and two unidentified species karyotyped: Hemipsilichthys sp. Paraitinga River (Kavalco et al., 2004, 2005), and Hemipsilichthys n. sp. Patos River (Alves et al., 2005). We suggest that further cytogenetic studies focus on expand the sampling in the northeast Brazil, the Western Amazon, the Guianas Shield, and other Neotropical countries, in addition to evaluate a more representative portion of the diversity.
Acknowledgments
The authors are grateful to all cytogeneticists who contributed to the increase of the knowledge about Loricariidae cytogenetics. We also thank to the Fundação de Amparo à Pesquisa do Estado de São Paulo (FMCS 2020/02681–9, 2022/04261–2, and 2023/08116–0; MBC AR-23/00955–2), to Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPQ (MBC 302928/2021–9), and Instituto Nacional de Ciência e Tecnologia da Biodiversidade e uso Sustentável de Peixes Neotropicais – INCT Peixes (405706/2022–7).
References
Almeida BRR, Souza LF, Alves TA, Cardoso AL, Oliveira JA, Ribas TFA et al. Chromosomal organization of multigene families and meiotic analysis in species of Loricariidae (Siluriformes) from Brazilian Amazon, with description of a new cytotype for genus Spatuloricaria. Biol Open. 2023; 12(11):bio060029. https://doi.org/10.1242/bio.060029
Alves AL, Borba RS, Pozzobon APB, Oliveira C, Nirchio M, Granado A et al. Localization of 18S ribosomal genes in suckermouth armoured catfishes Loricariidae (Teleostei, Siluriformes) with discussion on the Ag-NOR evolution. Comp Cytogen. 2012a; 6(3):315–21. https://doi.org/10.3897/CompCytogen.v6i3.2667
Alves AL, Borba RS, Oliveira C, Nirchio M, Granado A, Foresti F. Karyotypic diversity and evolutionary trends in the Neotropical catfish genus Hypostomus Lacépède, 1803 (Teleostei, Siluriformes, Loricariidae). Comp Cytogen. 2012b; 6(4):443–52. https://doi.org/10.3897/CompCytogen.v6i4.4028
Alves AL, Oliveira C, Foresti F. Karyotype variability in eight species of the subfamilies Loricariinae and Ancistrinae (Teleostei, Siluriformes, Loricariidae). Caryologia. 2003; 56(1):57–63. https://doi.org/10.1080/00087114.2003.10589308
Alves AL, Oliveira C, Foresti F. Comparative cytogenetic analysis of eleven species of subfamilies Neoplecostominae and Hypostominae (Siluriformes: Loricariidae). Genetica. 2005; 124:127–36. https://doi.org/10.1007/s10709-004-7561-4
Alves AL, Oliveira C, Nirchio M, Granado A, Foresti F. Karyotypic relationships among the tribes of Hypostominae (Siluriformes:Loricariidae) with description of XO sex chromosome system in a Neotropical fish species. Genetica. 2006; 128:1–09. https://doi.org/10.1007/s10709-005-0715-1
Andreata AA, Almeida-Toledo LF, Oliveira C, Toledo Filho SA. Chromosome Studies in Hypoptopomatinae (Pisces, Siluriformes, Loricariidae): I. XX/XY Sex Chromosome Heteromorphism in Pseudotocinclus tietensis. Cytologia. 1992; 57(3):369–72. https://doi.org/10.1508/cytologia.57.369
Andreata AA, Almeida-Toledo LF, Oliveira C, Toledo Filho SA. Chromosome studies in Hypoptopomatinae (Pisces, Siluriformes, Loricariidae) II. ZZ/ZW sex-chromosome system, B chromosomes, and constitutive heterochromatin differentiation in Microlepidogaster leucofrenatus. Cytogenet Genome Res. 1993; 63(4):215–20. https://doi.org/10.1159/000133538
Andreata AA, Almeida-Toledo LF, Oliveira C, Toledo Filho AS. Cytogenetic studies on the subfamily Hypoptopomatinae (Pisces, Siluriformes, Loricariidae). III. Analysis of seven species. Caryologia. 1994; 47(1):27–37. https://doi.org/10.1080/00087114.1994.10797279
Andreata AA, Ferreira DC, Foresti F, Oliveira C. Molecular cytogenetic study of heterochromatin in Hisonotus leucofrenatus (Teleostei, Loricariidae, Hypoptopomatinae). Hereditas. 2010; 147(1):10–17. https://doi.org/10.1111/j.1601-5223.2009.2149.x
Andreata AA, Oliveira C, Foresti F. Karyological characterization of four Neotropical fish species of the genus Hisonotus (Teleostei, Loricariidae, Hypoptopomatinae) from distinct Brazilian river basins. Genet Mol Biol. 2006; 29(1):62–66. https://doi.org/10.1590/S1415-47572006000100013
Anjos MS, Bitencourt JA, Nunes LA, Sarmento-Soares LM, Carvalho DC, Armbruster JW et al. Species delimitation based on integrative approach suggests reallocation of genus in Hypostomini catfish (Siluriformes, Loricariidae). Hydrobiologia. 2020; 847:563–78. https://doi.org/10.1007/s10750-019-04121-z
Arai R. Fish karyotypes a check list. Springer: Tokyo, Japan; 2011.
Arratia G, Kapoor BG, Chardon M, Diogo R. Catfishes Vol. 1 & 2. Enfield, NH: Science Publishers; 2003.
Artoni RF, Bertollo LAC. Cytogenetic studies on Hypostominae (Pisces, Siluriformes, Loricariidae). Considerations on karyotype evolution in the genus Hypostomus. Caryologia. 1996; 49(1):81–90. https://doi.org/10.1080/00087114.1996.10797353
Artoni RF, Bertollo LAC. Nature and distribution of constitutive heterochromatin in fishes, genus Hypostomus (Loricariidae). Genetica. 1999; 106:209–14. https://doi.org/10.1023/A:1003957719178
Artoni RF, Bertollo LAC. Trends in the karyotype evolution of Loricariidae fish (Siluriformes). Hereditas. 2001; 134(3):201–10. https://doi.org/10.1111/j.1601-5223.2001.00201.x
Artoni RF, Venere PC, Bertollo LAC. A heteromorphic ZZ/ZW sex chromosome system in fish, genus Hypostomus (Loricariidae). Cytologia. 1998; 63(4):421–25. https://doi.org/10.1508/cytologia.63.421
Ayres-Alves T, Cardoso AL, Nagamachi CY, Sousa LM, Pieczarka JC, Noronha RCR. Karyotypic evolution and chromosomal organization of repetitive DNA sequences in species of Panaque, Panaqolus, and Scobinancistrus (Siluriformes and Loricariidae) from the Amazon basin. Zebrafish. 2017; 14(3):251–60. https://doi.org/10.1089/zeb.2016.1373
Barros AV, Wolski MAV, Nogaroto V, Almeida MC, Moreira-Filho O, Vicari MR. Fragile sites, dysfunctional telomere and chromosome fusions: what is 5S rDNA role? Gene. 2017; 608:20–27. https://doi.org/10.1016/j.gene.2017.01.013
Barros FJT, Rodrigues ELC, Moura MCS, Torres RA, Paula EA, Sousa LM. Weight-length and length-length relationships of the endangered zebra pleco Hypancistrus zebra (Siluriformes, Loricariidae) from the Xingu River, Amazon, Brazil. J Appl Ichthyol. 2023; 5158180. https://doi.org/10.1155/2023/5158180
Baumgärtner L, Paiz LM, Zawadzki CH, Margarido VP, Castro ALBP. Heterochromatin polymorphism and physical mapping of 5S and 18S ribosomal DNA in four populations of Hypostomus strigaticeps (Regan, 1907) from the Paraná River basin, Brazil: evolutionary and environmental correlation. Zebrafish. 2014; 11(5):479–87. https://doi.org/10.1089/zeb.2014.1028
Becker QMC, Castro RJ, Silva AM, Vizzotto PC. Cytogenetic characterization of two species of Hypostomus (Siluriformes, Loricariidae) from tributaries of the Vermelho river, upper Paraguay river basin. Biodiversidade. 2014; 13(1):1–13.
Benitez MF, Pastori MC, Garrido GG, Takagui F, Giuliano L, Fenocchio AS. First cytogenetic characterization of Loricaria simillima (Loricariidae, Siluriformes) from Paraná River (Argentina) with emphasis in cytotaxonomy of Loricaria. Caryologi. 2017; 70(1):29–33. https://doi.org/10.1080/00087114.2016.1258158
Bitencourt JA, Affonso PRAM, Giuliano-Caetano L, Dias AL. Heterochromatin heterogeneity in Hypostomus prope unae (Steindachner, 1878) (Siluriformes, Loricariidae) from Northeastern Brazil. Comp Cytogen. 2011a; 5(4):329–44. https://doi.org/10.3897/CompCytogen.v5i4.1149
Bitencourt JA, Affonso PRAM, Giuliano-Caetano L, Dias AL. Identification of distinct evolutionary units in allopatric populations of Hypostomus cf. wuchereri Günther, 1864 (Siluriformes: Loricariidae): karyotypic evidence. Neotrop Ichthyol. 2011b; 9(2):317–24. https://doi.org/10.1590/S1679-62252011000200008
Bitencourt JA, Affonso PRAM, Giuliano-Caetano L, Carneiro PLS, Dias AL. Population divergence and peculiar karyoevolutionary trends in the loricariid fish Hypostomus aff. unae from northeastern Brazil. Genet Mol Res. 2012; 11(2):933–43. http://doi.org/10.4238/2012.April.13.1
Blanco DR, Vicari MR, Artoni RF, Traldi JB, Moreira-Filho O. Chromosomal characterization of armored catfish Harttia longipinna (Siluriformes, Loricariidae): first report of B chromosomes in the genus. Zool Sci. 2012; 29(9):604–09. http://doi.org/10.2108/zsj.29.604
Blanco DR, Vicari MR, Lui RL, Bertollo LAC, Traldi JB, Moreira-Filho O. The role of the Robertsonian rearrangements in the origin of the XX/XY1Y2 sex chromosome system and in the chromosomal differentiation in Harttia species (Siluriformes, Loricariidae). Rev Fish Biol Fish. 2013; 23(1):127–34. http://doi.org/10.1007/s11160-012-9283-5
Blanco DR, Vicari MR, Lui RL, Artoni RF, Almeida MC, Traldi JB et al. Origin of the X1X1X2X2/X1X2Y sex chromosome system of Harttia punctata (Siluriformes, Loricariidae) inferred from chromosome painting and FISH with ribosomal DNA markers. Genetica. 2014; 142(2):119–26. http://doi.org/10.1007/s10709-014-9759-4
Blanco DR, Vicari MR, Lui RL, Traldi JB, Bueno V, Martinez JF et al. Karyotype diversity and evolutionary trends in armored catfish species of the genus Harttia (Siluriformes: Loricariidae). Zebrafish. 2017; 14(2):169–76. http://doi.org/10.1089/zeb.2016.1377
Brandão KO, Rocha-Reis DA, Garcia C, Pazza R, Almeida-Toledo LF, Kavalco KF. Studies in two allopatric populations of Hypostomus affinis (Steindachner, 1877): the role of mapping the ribosomal genes to understand the chromosome evolution of the group. Comp Cytogen. 2018; 12(1):1–12. http://doi.org/10.3897/CompCytogen.v12i1.22052
Briggs JC. The biogeography of Otophysan fishes (Ostariophysi: Otophysi): a new appraisal. J Biogeography. 2005; 32(2):287–94. https://doi.org/10.1111/j.1365-2699.2004.01170.x
Buck S, Sazima I. An assemblage of mailed catfishes (Loricariidae) in southeastern Brazil: distribution, activity and feeding. Ichthyol Explor Freshw. 1995; 6(4):325–32.
Bueno V, Zawadzki CH, Margarido VP. Trends in chromosome evolution in the genus Hypostomus Lacépède, 1803 (Osteichthyes, Loricariidae): a new perspective about the correlation between diploid number and chromosomes types. Rev Fish Biol Fish. 2012; 22:241–50. http://doi.org/10.1007/s11160-011-9215-9
Bueno V, Venere PC, Zawadzki CH, Margarido VP. Karyotypic diversification in Hypostomus Lacépède, 1803 (Siluriformes, Loricariidae): biogeographical and phylogenetic perspectives. Rev Fish Biol Fish. 2013; 23(1):103–12. http://doi.org/10.1007/s11160-012-9280-8
Bueno V, Venere PC, Konerat JT, Zawadzki CH, Vicari MR, Margarido VP. Physical mapping of the 5S and 18S rDNA in ten species of Hypostomus Lacépède 1803 (Siluriformes: Loricariidae): evolutionary tendencies in the genus. Sci World J. 2014; 943825. http://doi.org/10.1155/2014/943825
Bueno V, Konerat JT, Zawadzki CH, Venere PC, Blanco DR, Margarido VP. Divergent chromosome evolution in Hypostominae tribes (Siluriformes: Loricariidae): correlation of chromosomal data with morphological and molecular phylogenies. Zebrafish. 2018; 15(5):492–503. http://doi.org/10.1089/zeb.2018.1612
Camilo FM, Moreira-Filho O. Karyotypic description of Corumbataia cuestae (Pisces, Loricariidae, Hypoptopomatinae). Cytologia. 2005; 70(1):47–51. https://doi.org/10.1508/cytologia.70.47
Campos H, Arratia G, Cuevas C. Karyotypes of the most primitive catfishes (Teleostei: Siluriformas: Diplomystidae). J Zool System Evol Res. 1997; 35:113–19. https://doi.org/10.1111/j.1439-0469.1997.tb00412.x
Cardoso AL, Carvalho HLS, Benathar TCM, Serrão SMG, Nagamachi CY, Pieczarka JC et al. Integrated cytogenetic and mitochondrial DNA analyses indicate that two different phenotypes of Hypancistrus (L066 and L333) belong to the same species. Zebrafish. 2016; 13(3):209–16. https://doi.org/10.1089/zeb.2015.1213
Cardoso AL, Sales KAH, Nagamachi CY, Pieczarka JC, Noronha RCR. Comparative cytogenetics of two species of genus Scobinancistrus (Siluriformes, Loricariidae, Ancistrini) from the Xingu River, Brazil. Comp Cytogen. 2013; 7(1):43–51. https://doi.org/10.3897/CompCytogen.v7i1.4128
Carvalho ML, Silva GJC, Melo S, et al. The non-monotypic status of the Neotropical fish genus Hemiodontichthys (Siluriformes, Loricariidae) evidenced by genetic approaches. Mitochondrial DNA Part A. 2018; 29(8):1224–30. https://doi.org/10.1080/24701394.2018.1431230
Cassemiro FAS, Albert JS, Antonelli A, Graham CH. Landscape dynamics and diversification of the megadiverse South American freshwater fish fauna. PNAS. 2023, 120(2):e2211974120. https://doi.org/10.1073/pnas.2211974120
Centofante L, Bertollo LAC, Moreira-Filho O. Cytogenetic characterization and description of an XX/XY1Y2 sex chromosome system in catfish Harttia carvalhoi (Siluriformes, Loricariidae). Cytogen Genome Res. 2006; 112(3–4):320–24. https://doi.org/10.1159/000089887
Centofante L, Vicari MR, Artoni RF, Buckup PA, Bertollo LAC, Moreira-Filho O. Chromosome analyses in species of Pareiorhina (Siluriformes, Loricariidae) and cytotaxonomic considerations on the group. Nucleus. 2011; 54(2):65–70. https://doi.org/10.1007/s13237-011-0035-z
Cereali SS, Pomini E, Rosa R, Zawadzki CH, Froehlich O, Giuliano-Caetano L. Karyotype description of two species of Hypostomus (Siluriformes, Loricariidae) of the Planalto da Bodoquena, Brazil. Genet Mol Res. 2008; 7(3):583–91. https://doi.org/10.4238/vol7-3gmr404
Cius A, Lorscheider CA, Barbosa LM, Zawadzki CH, Borin-Carvalho LA, Porto FE et al. Contributions of species Rineloricaria pentamaculata (Loricariidae:Loricariinae) in a karyoevolutionary context. Caryologia. 2022; 75(3):39–46. https://doi.org/10.36253/caryologia-1611
Cristina FD, Chiachio MC, Takako AK, Andreata AA, Foresti F, Oliveira C. Comparative cytogenetics of nine species of Hypoptopomatinae (Teleostei: Siluriformes: Loricariidae): the importance of structural rearrangements in chromosome evolution. Caryologia. 2005; 58(4):387–95. https://doi.org/10.1080/00087114.2005.10589478
Deon GA, Glugoski L, Vicari MR, Nogaroto V, Sassi FMC, Cioffi MB et al. Highly rearranged karyotypes and multiple sex chromosome systems in armored catfishes from the genus Harttia (Teleostei, Siluriformes). Genes. 2020; 11(11):1366. https://doi.org/10.3390/genes11111366
Deon GA, Glugoski L, Sassi FMC, Hatanaka T, Nogaroto V, Bertollo LAC et al. Chromosomal rearrangements and origin of the multiple XX/XY1Y2 sex chromosome system in Harttia Species (Siluriformes: Loricariidae). Front Genet. 2022a; 13:877522. https://doi.org/10.3389/fgene.2022.877522
Deon GA, Glugoski L, Hatanaka T, Sassi FMC, Nogaroto V, Bertollo LAC et al. Evolutionary breakpoint regions and chromosomal remodeling in Harttia (Siluriformes: Loricariidae) species diversification. Genet Mol Biol. 2022b; 45(2):e20210170. https://doi.org/10.1590/1678-4685-GMB-2021-0170
Endo KS, Martinez ERM, Zawadzki CH, Paiva LRS, Júlio Junior HF. Karyotype description of possible new species of the Hypostomus ancistroides complex (Teleostei: Loricariidae) and other Hypostominae. Acta Scient Biol Sci. 2012; 34(2):181–89. https://doi.org/10.4025/actascibiolsci.v34i2.9318
Evers H, Pinnegar JK, Taylor MI. Where are they all from? – sources and sustainability in the ornamental freshwater fish trade. J Fish Biol. 2019; 94(6):909–16. https://doi.org/10.1111/jfb.13930
Favarato RM, Silva M, Oliveira RR, Artoni RF, Feldberg E, Matoso DA. Cytogenetic diversity and the evolutionary dynamics of rDNA genes and telomeric sequences in the Ancistrus genus (Loricariidae: Ancistrini). Zebrafish. 2016; 13(2):103–11. https://doi.org/10.1089/zeb.2015.1140
Fenocchio AS, Pastori MC, Roncati HÁ, Moreira-Filho O, Bertollo LAC. A cytogenetic survey of the fish fauna from Argentina. Caryologia. 2003; 56(2):197–204. https://doi.org/10.1080/00087114.2003.10589325
Fernandes CA, Alves DS, Guterres ZR, Martins-Santos IC. Cytogenetic analysis of two locariid species (Teleostei, Siluriformes) from Iguatemi River (Paraná River drainage) in Brazil. Comp Cytogenet. 2015; 9(1):67–78. https://doi.org/10.3897/CompCytogen.v9i1.8804
Fernandes CA, Bailly D, Sant’Ana DRA, Alves DS. First cytogenetic record for a species of Otothyropsis Ribeiro, Carvalho & Melo, 2005 (Loricariidae, Hypoptopomatinae). Neotrop Ichthyol. 2016, 14(1):e150123. https://doi.org/10.1590/1982-0224-20150123
Fernandes CA, Damásio JF, Martins-Santos IC. Cytogenetic studies in species of family Loricariidae (Osteichthyes, Siluriformes) from Iguatemi river basin, Brazil. First cytogenetic report in Farlowella amazonum (Günther, 1864). Caryologia. 2012; 65(4):276–80. https://doi.org/10.1080/00087114.2012.752913
Fernandes CA, Paiz LM, Piscor D, Gavazzoni M, Carvalho LAB, Portela-Castro ALB et al. Chromosomal diversity in two allopatric populations of Farlowella hahni Meinken 1937 (Teleostei: Siluriformes): cytogenetics and cytochrome b analyses. Zebrafish. 2021; 18(1):66–72. https://doi.org/10.1089/zeb.2020.1966
Fernandino JI, Hattori RS. Sex determination in Neotropical fish: Implications ranging from aquaculture technology to ecological assessment. Gen Comp Endocrinol. 2019; 273:172–83. https://doi.org/10.1016/j.ygcen.2018.07.002
Ferreira AMV, Viana PF, Zuanon J,Ezaz T, Cioffi MB, Takagui FH et al. Cytogenetic analysis of Panaqolus tankei Cramer & Sousa, 2016 (Siluriformes, Loricariidae), an ornamental fish endemic to Xingu River, Brazil. Cytogenet Genome Res. 2021; 161(3–4):187–94. https://doi.org/10.1159/000514061
Ferreira GEB, Barbosa LM, Prizon-Nakajima AC, Paiva S, Vieira MMR, Gallo RB et al. Constitutive heterochromatin heteromorphism in the Neotropical armored catfish Hypostomus regani (Ihering, 1905) (Loricariidae, Hypostominae) from the Paraguay River basin (Mato Grosso do Sul, Brazil). Comp Cytogenet. 2019; 13(1):27–39. https://doi.org/10.3897/CompCytogen.v13i1.30134
Ferreira RO, Pereira AL, Nagamachi CY, Pieczarka JC, Sousa LM, Noronha RCR. Caracterização citogenética de uma espécie de Spatuloricaria (Siluriformes, Loricariidae) do rio Xingu, (Pará, Amazônia, Brasil). Biota Amazôn. 2014; 4(1):30–36. http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v4n1p30-36
Fink S, Fink W. Interrelationships of the ostariophysan fishes (Teleostei). ZJLS. 1981; 72:297–353.
Fink S, Fink W. Interrelationships of ostariophysan fishes (Teleostei). In: Stiassny MLJ, Parenti LR, Johnson GD, editors. Interrelationships of fishes. New York: Academic Press; 1996. p.209–45.
Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2023. Available from: https://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Glugoski L, Giuliano-Caetano L, Moreira-Filho O, Vicari MR, Nogaroto V. Co-located hAT transposable element and 5S rDNA in an interstitial telomeric sequence suggest the formation of Robertsonian fusion in armored catfish. Gene. 2018; 650:49–54. https://doi.org/10.1016/j.gene.2018.01.099
Glugoski L, Deon G, Schott S, Vicari MR, Nogaroto V, Moreira-Filho O. Comparative cytogenetic analyses in Ancistrus species (Siluriformes: Loricariidae). Neotrop Ichthyol. 2020; 18(2):e200013. https://doi.org/10.1590/1982-0224-2020-0013
Glugoski L, Nogaroto V, Deon GA, Azambuja M, Moreira-Filho O, Vicari MR. Enriched tandem repeats in chromosomal fusion points of Rineloricaria latirostris (Boulenger, 1900) (Siluriformes: Loricariidae). Genome. 2022; 65(9):479–89. https://dx.doi.org/10.1139/gen-2022-0043
Glugoski L, Deon GA, Nogaroto V, Moreira-Filho O, Vicari MR. Robertsonian fusion site in Rineloricaria pentamaculata (Siluriformes: Loricariidae): involvement of 5S rDNA and satellite sequences. Cytogenet Genome Res. 2023; 162(11–12):657–64. https://doi.org/10.1159/000530636
Guimarães AS, Maciel LAM, Souza MFB, Rodrigues LRR. Karyotypic and molecular analysis of Pterygoplichthys pardalis (Castelnau 1855) from the lower Amazon River. Animals. 2023; 13(9):1533. https://doi.org/10.3390/ani13091533
Hinegardner R, Rosen DE. Cellular DNA content and the evolution of teleostean fishes. The American Naturalist. 1972; 106(951):621–44. https://doi.org/10.1086/282801
Kamei MCSL, Baumgärtner L, Paiva S, Zawadzki CH, Martins-Santos IC, Portela-Castro ALB. Chromosomal diversity of three species of Hypostomus Lacépède, 1803 (Siluriformes, Loricariidae), from the Paraná River basin, Brazil: a species complex in Hypostomus ancistroides Reinforced by a ZZ/ZW sex chromosome system. Zebrafish. 2017; 14(4):357–63. https://doi.org/10.1089/zeb.2017.1429
Kavalco KF, Pazza R, Bertollo LAC, Moreira-Filho O. Heterochromatin characterization of four fish species of the family Loricariidae (Siluriformes). Hereditas. 2004; 141(3):237–42. https://doi.org/10.1111/j.1601-5223.2004.01850.x
Kavalco KF, Pazza R, Bertollo LAC, Moreira-Filho O. Karyotypic diversity and evolution of Loricariidae (Pisces, Siluriformes). Heredity. 2005; 94(2):180–86. https://doi.org/10.1038/sj.hdy.6800595
Konerat JT, Bueno V, Margarido VP, Portela-Castro ALB, Martins-Santos IC. Diversity of sex chromosome systems in Ancistrini (Loricariidae, Hypostominae): ZZ/ZW in Ancistrus taunayi Miranda Ribeiro, 1918. Cytogenet Genome Res. 2015; 146(4):306–10. https://doi.org/10.1159/000441431
Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964; 52:201–20.
Lévêque C, Oberdorff T, Paugy D, Stassny MLJ, Tedesco PA. Global diversity of fish (Pisces) in freshwater. Hydrobiologia. 2008; 595:545–67. https://doi.org/10.1007/s10750-007-9034-0
Lorscheider CA, Oliveira JIN, Dulz TA,Nogaroto V, Martins-Santos IC, Vicari MR. Comparative cytogenetics among three sympatric Hypostomus species (Siluriformes: Loricariidae): an evolutionary analysis in a high endemic region. Braz Arch Biol Technol. 2018; 61:e18180417. http://dx.doi.org/10.1590/1678-4324-2018180417
Lorscheider CA, Zawadzki CH, Vicari MR, Martins-Santos IC, Artoni RF. Karyotypic diversity of the armoured catfish genus Hypostomus (Siluriformes: Loricariidae) in the context of its occurrence and distribution. J Fish Biol. 2015; 87(4):1099–110. https://doi.org/10.1111/jfb.12762
Lujan NK, Armbruster JW, Lovejoy NR, López-Fernández H. Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Mol Phylogenet Evol. 2015; 82:269–88. https://doi.org/10.1016/j.ympev.2014.08.020
Lujan NK, Cramer CA, Covain R, Fisch-Muller S, López-Fernández H. Multilocus molecular phylogeny of the ornamental wood-eating catfishes (Siluriformes, Loricariidae, Panaqolus and Panaque) reveals undescribed diversity and parapatric clades. Mol Phylogenet Evol. 2017; 109:321–36. https://doi.org/10.1016/j.ympev.2016.12.040
Lujan NK, Hidalgo M, Stewart DJ. Revision of Panaque (Panaque), with descriptions of three new species from the Amazon Basin (Siluriformes, Loricariidae). Copeia. 2010; 4(2010):676–704. https://doi.org/10.1643/CI-09-185
Maia TPA, Giuliano-Caetano L, Rodriguez MS, Rubert M, Takagui FH, Dias AL. Chromosomal banding in three species of the genus Rineloricaria (Siluriformes, Loricariidae, Loricariinae). Ichthyol Res. 2010; 57(2):209–13. https://doi.org/10.1007/s10228-009-0145-7
Marajó L, Viana PF, Ferreira M, Rapp Py-Daniel LH, Feldberg E. Cytogenetics of two Farlowella species (Loricariidae: Loricariinae): implications on the taxonomic status of the species. Netrop Ichthyol. 2018; 16(4):e180029. https://doi.org/10.1590/1982-0224-20180029
Marajó L, Viana PF, Ferreira AMV, Rapp Py-Daniel LH, Cioffi MB, Sember A et al. Chromosomal rearrangements and the first indication of an ♀X1X1X2X2/♂X1X2Y sex chromosome system in Rineloricaria fishes (Teleostei: Siluriformes). J Fish Biol. 2022; 102(2):443–54. https://doi.org/10.1111/jfb.15275
Mariotto S, Artoni RF, Miyazawa CS. Occurrence of sexual chromosome, of the type ZZ/ZW, in Ancistrus cf. dubius (Loricariidae, Ancistrinae) of the Paraguay River Basin, Mato Grosso, Brazil. Caryologia. 2004; 57(4):327–31. https://doi.org/10.1080/00087114.2004.10589413
Mariotto S, Centofante L, Miyazawa CS, Bertollo LAC, Moreira-Filho O. Chromosome polymorphism in Ancistrus cuiabae Knaack, 1999 (Siluriformes: Loricariidae: Ancistrini). Netrop Ichthyol. 2009; 7(4):595–600. https://doi.org/10.1590/S1679-62252009000400006
Mariotto S, Centofante L, Moreira-Filho O. Diversity and chromosomal evolution in the genus Ancistrus Kner, 1854 (Loricariidae: Ancistrini) from three hydrographic basins of Mato Grosso State, Brazil. Netrop Ichthyol. 2013; 11(1):125–31. https://doi.org/10.1590/S1679-62252013000100015
Mariotto S, Centofante L, Venere PC, Ferreira DC, Artoni RF. New comparative cytogenetic data on three genera of armored catfishes of Ancistrini (Loricariidae: Hypostominae). Cytogenet Genome Res. 2019; 159(4):208–14. https://doi.org/10.1159/000504723
Mariotto S, Centofante L, Vicari MR, Artoni RF, Moreira-Filho O. Chromosomal diversification in ribosomal DNA sites in Ancistrus Kner, 1854 (Loricariidae, Ancistrini) from three hydrographic basins of Mato Grosso, Brazil. Comp Cytogenet. 2011; 5(4):289–300. https://doi.org/10.3897/CompCytogen.v5i4.1757
Mariotto S, Miyazawa CS. Ancistrus cf. dubius (Siluriformes, Ancistrinae), a complex of species. 1. Chromosomic characterization of four populations and occurence of sexual chromosomes of type XX/XY, in the Pantanal basin of Mato Grosso, Brazil. Caryologia. 2006, 59(4):299–304. https://doi.org/10.1080/00087114.2006.10797929
Martinez ERM, Zawadzki CH, Foresti F, Oliveira C. Cytogenetic analysis of five Hypostomus species (Siluriformes, Loricariidae). Genet Mol Biol. 2011; 34(4):562–68. https://doi.org/10.1590/S1415-47572011005000038
Maurutto FAM, Manvailer LFS, Sczepanski TS, Cestari MM, Artoni RF. Cytogenetic characterization of three allopatric species of Hypostomus Lacépède (1803) (Teleostei, Loricariidae). Caryologia. 2012; 65(4):340–46. https://doi.org/10.1080/00087114.2012.760882
Medeiros LA, Ginani EG, Sousa LM, Rapp Py-Daniel LH, Feldberg E. Cytogenetic analysis of Baryancistrus xanthellus (Siluriformes: Loricariidae: Ancistrini), an ornamental fish endemic to the Xingu River, Brazil. Neotrop Ichthyol. 2016; 14(2):e150108. https://doi.org/10.1590/1982-0224-20150108
Mendes-Neto EO, Vicari MR, Artoni RF, Moreira-Filho O. Description of karyotype in Hypostomus regani (Ihering, 1905) (Teleostei, Loricariidae) from the Piumhi river in Brazil with comments on karyotype variation found in Hypostomus. Comp Cytogenet. 2011; 5(2):133–42. https://doi.org/10.3897/CompCytogen.v5i2.964
Michele JL, Takahashi CS, Ferrari I. Karyotypic study of some species of the family Loricariidae. Cytologia. 1977; 42:539–46. https://doi.org/10.1508/cytologia.42.539
Milhomem SSR, Castro RR, Nagamachi CY, Souza A, Feldberg E, Pieczarka J. Different cytotypes in fishes of the genus Hypostomus Lcépède, 1803, (Siluriformes: Loricariidae) from Xingu river (Amazon region, Brazil). Comp Cytogenet. 2010; 4(1):45–54. https://doi.org/10.3897/compcytogen.v4i1.31
Muramoto J, Ohno S, Atkin NB. On the diploid state of the fish Order Ostariophysi. Chromosoma. 1968; 24:59–66. https://doi.org/10.1007/BF00329607
Nelson JS, Grande TC, Wilson MV. Fishes of the world. Hoboken, New Jersey: John Wiley & Sons; 2016.
Nirchio M, Oliveira C, Cioffi MB, Sassi FMC, Valdiviezo J, Paim FG et al. Occurrence of sex chromosomes in fish of the genus Ancistrus with a new description of multiple sex chromosomes in the Ecuadorian endemic Ancistrus clementinae (Loricariidae). Genes. 2023; 14:306. https://doi.org/10.3390/genes14020306
Novák J, Hofmann J, Hohl D, Magalhães ALB, Patoka J. Enigmatic armoured catfishes (Siluriformes: Callichthyidae and Loricariidae) in ornamental aquaculture: a new insight into Neotropical fish diversity. Aquaculture. 2022; 547:737460. https://doi.org/10.1016/j.aquaculture.2021.737460
Novák J, Kalous L, Patoka J. Modern ornamental aquaculture in Europe: early history of freshwater fish imports. Rev Aquaculture. 2020; 12(4):2042–60. https://doi.org/10.1111/raq.12421
Oliveira C, Gosztonyi AE. A cytogenetic study of Diplotnystes mesembrinus (Teleostei, Siluriformes, Diplomystidae) with a discussion of chromosome evolution in siluriforms. Caryologia. 2000; 53(1):31–37. https://doi.org/10.1080/00087114.2000.10589178
Oliveira LC, Ribeiro MO, Costa GM, Zawadzki CH, Prizon-Nakajima AC, Borin-Carvalho LA et al. Cytogenetic characterization of Hypostomus soniae Hollanda-Carvalho & Weber, 2004 from the Teles Pires River, southern Amazon basin: evidence of an early stage of an XX/XY sex chromosome system. Comp Cytogenet. 2019; 13(4):411–22. https://doi.org/10.3897/CompCytogen.v13i4.36205
Oliveira LC, Ribeiro MO, Dutra ES, Zawadzki CH, Portela-Castro ALB, Martins-Santos IC. Karyotype structure of Hypostomus cf. plecostomus (Linnaeus, 1758) from Tapajós River basin, Southern Amazon: occurrence of sex chromosomes (ZZ/ZW) and their evolutionary implications. Genet Mol Res. 2015; 14(2):6625–34. http://dx.doi.org/10.4238/2015.June.18.5
Oliveira RR, Feldberg E, Anjos MB, Zuanon J. Mechanisms of chromosomal evolution and its possible relation to natural history characteristics in Ancistrus catfishes (Siluriformes: Loricariidae). J Fish Biol. 2009; 75(9):2209–25. https://doi.org/10.1111/j.1095-8649.2009.02450.x
Oliveira RR, Feldberg E, Anjos MB, Zuanon J. Karyotype characterization and ZZ/ZW sex chromosome heteromorphism in two species of the catfish genus Ancistrus Kner, 1854 (Siluriformes: Loricariidae) from the Amazon basin. Neotrop Ichthyol. 2007; 5(3):301–06. https://doi.org/10.1590/S1679-62252007000300010
Oliveira RR, Feldberg E, Anjos MB, Zuanon J. Occurrence of multiple sexual chromosomes (XX/XY1Y2 and Z1Z1Z2Z2/Z1Z2W1W2) in catfishes of the genus Ancistrus (Siluriformes: Loricariidae) from the Amazon basin. Genetica. 2008; 134(2):243–49. https://doi.org/10.1007/s10709-007-9231-9
Oliveira RR, Souza IL, Venere PC. Karyotype description of three species of Loricariidae (Siluriformes) and occurrence of the ZZ/ZW sexual system in Hemiancistrus spilomma Cardoso & Lucinda, 2003. Neotrop Ichthyol. 2006; 4(1):93–97. https://doi.org/10.1590/S1679-62252006000100010
Pansonato-Alves JC, Serrano EA, Utsunomia R, Scacchetti P, Oliveira C, Foresti F. Mapping five repetitive DNA classes in sympatric species of Hypostomus (Teleostei: Siluriformes: Loricariidae): analysis of chromosomal variability. Rev Fish Biol Fish. 2013; 23:477–89. https://doi.org/10.1007/s11160-013-9303-0
Paula GBN, Gavazzoni M, Zawadzki CH, Fernandes CA, Portela_castro ALB, Lui RL et al. Identification of cryptic species in allopatric populations of Hypostomus tietensis (Siluriformes: Loricariidae) through cytogenetics analyses. Neotrop Ichthyol. 2022; 20(2):e210158. https://doi.org/10.1590/1982-0224-2021-0158
Pazian MF, Pereira LHG, Shimabukuru-Dias CK, Oliveira C, Foresti F. Cytogenetic and molecular markers reveal the complexity of the genus Piabina Reinhardt, 1867 (Characiformes: Characidae). Neotrop Ichthyol. 2012; 10(2):329–40. https://doi.org/10.1590/S1679-62252012005000015
Pereira EH, Reis RE. Morphology-based phylogeny of the suckermouth armored catfishes, with emphasis on the Neoplecostominae (Teleostei: Siluriformes: Loricariidae). Zootaxa. 2017; 4264(1):1–104. https://doi.org/10.11646/zootaxa.4264.1.1
Pety AM, Cardoso AL, Nagamachi CY, Pieczarka JC, Sousa LM, Rodrigues RC. In situ localization of ribosomal sites in Peckoltia and Ancistomus (Loricariidae: Hypostominae) from the Amazon basin. Zebrafish. 2018; 15(3):263–69. https://doi.org/10.1089/zeb.2017.1523
Porto FE, Portela-Castro ALB, Martins-Santos IC. Possible origins of B chromosomes in Rineloricaria pentamaculata (Loricariidae, Siluriformes) from the Paraná River basin. Genet Mol Res. 2010; 9(3):1654–59. https://doi.org/10.4238/vol9-3gmr859
Porto FE, Portela-Castro ALB, Martins-Santos IC. Chromosome polymorphism in Rineloricaria pentamaculata (Loricariidae, Siluriformes) of the Paraná River basin. Ichthyol Res. 2011; 58:225–31. https://doi.org/10.1007/s10228-011-0215-5
Porto FE, Vieira MMR, Barbosa LM, Borin-Carvalho LA, Vicari MR, Portela-Castro ALB et al. Chromosomal polymorphism in Rineloricaria lanceolata Günther, 1868 (Loricariidae: Loricariinae) of the Paraguay basin (Mato Grosso do Sul, Brazil): evidence of fusions and their consequences in the population. Zebrafish. 2014a; 11(4):318–24. https://doi.org/10.1089/zeb.2014.0996
Porto FE, Gindri BS, Vieira MMR, Borin LA, Portela-Castro ALB,Martins-Santos IC. Polymorphisms of the nucleolus organizing regions in Loricaria cataphracta (Siluriformes, Loricariidae) of the upper Paraguay River basin indicate an association with transposable elements. Genet Mol Res. 2014b; 13(1):1627–34. http://dx.doi.org/10.4238/2014.March.12.15
Price AC, Weadick CJ, Shim J, Rodd FH. Pigments, patterns, and fish behavior. Zebrafish. 2008; 5(4):297–307. https://doi.org/10.1089/zeb.2008.0551
Primo CC, Glugoski L, Almeida MC, Zawadzki CH, Moreira-Filho O, Vicari MR et al. Mechanisms of chromosomal diversification in species of Rineloricaria (Actinopterygii: Siluriformes: Loricariidae). Zebrafish. 2017; 14(2):161–68. https://doi.org/10.1089/zeb.2016.1386
Primo CC, Glugoski L, Vicari MR, Nogaroto V. Chromosome mapping and molecular characterization of the Tc1/Mariner element in Rineloricaria (Siluriformes: Loricariidae). Braz Arch Biol Technol. 2018; 61:e18170623. http://dx.doi.org/10.1590/1678-4324-2018170623
Prizon AC, Borin-Carvalho LA, Bruschi DP, Ribeiro MO, Barbosa LM, Ferreira GEB et al. Cytogenetic data on Ancistrus sp. (Siluriformes, Loricariidae) of the Paraguay River basin (MS) sheds light on intrageneric karyotype diversification. Comp Cytogenet. 2016; 10(4):625–36. https://doi.org/10.3897/CompCytogen.v10i4.8532
Prizon AC, Bruschi DP, Borin-Carvalho LA, Cius A, Barbosa LM, Ruiz HB et al. Hidden diversity in the populations of the armored catfish Ancistrus Kner, 1854 (Loricariidae, Hypostominae) from the Paraná River basin revealed by molecular and cytogenetic data. Front Genet. 2017; 8:185. https://doi.org/10.3389/fgene.2017.00185
Ramirez JL, Birindelli JL, Carvalho DC, Affonso PRAM, Venere PC, Ortega H et al. Revealing hidden diversity of the underestimated Neotropical ichthyofauna: DNA barcoding in the recently described genus Megaleporinus (Characiformes:Anostomidae). Front Genet. 2017; 8:149. https://doi.org/10.3389/fgene.2017.00149
Ribeiro AC, Lima FCT, Pereira EHL. A new genus and species of a minute suckermouth armored catfish (Siluriformes: Loricariidae) from the rio Tocantins drainage, Central Brazil: the smallest known loricariid catfish. Copeia. 2012; 2012(4):637–47. https://doi.org/10.1643/CI-11-137
Ribeiro MO, Noleto RB, Lorscheider CA, Porto FE, Prizon AC, Zawadzki CH et al. Cytogenetic description of Ancistrus abilhoai (Siluriformes: Loricariidae) from Iguaçu River basin, southern Brazil. Genet Mol Res. 2015; 14(2):4051–57. http://dx.doi.org/10.4238/2015.April.27.20
Rocha-Reis D, Brandão KO, Almeida-Toledo LF, Pazza R, Kavalco KF. The persevering cytotaxonomy: discovery of a unique XX/XY sex chromosome system in catfishes suggests the existence of a new, endemic and rare species. Cytogenet Gen Res. 2018; 156(1):45–55. https://doi.org/10.1159/000492959
Rosa KO, Ziemniczak K, Barros AV, Nogaroto V, Almeida M, Cestari MM et al. Numeric and structural chromosome polymorphism in Rineloricaria lima (Siluriformes: Loricariidae): fusion points carrying 5S rDNA or telomere sequence vestiges. Rev Fish Biol Fish. 2012; 22(3):739–49. https://doi.org/10.1007/s11160-011-9250-6
Roxo FF, Albert JS, Silva GS, Zawadzki CH, Foresti F, Oliveira C. Molecular phylogeny and biogeographic history of the armored Neotropical catfish subfamilies Hypoptopomatinae, Neoplecostominae and Otothyrinae (Siluriformes: Loricariidae). PLoS ONE. 2014; 9(8):e105564. https://doi.org/10.1371/journal.pone.0105564
Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC et al. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phylogenet Evol. 2019; 135:148–65. https://doi.org/10.1016/j.ympev.2019.02.017
Rubert M, Rosa R, Jerep FC, Bertollo LAC, Giuliano-Caetano L. Cytogenetic characterization of four species of the genus Hypostomus Lacépède, 1803 (Siluriformes, Loricariidae) with comments on its chromosomal diversity. Comp Cytogenet. 2011; 5(5):397–410. https://doi.org/10.3897/CompCytogen.v5i5.1589
Rubert M, Rosa R, Zawadzki CH, Mariotto S, Moreira-Filho O, Giuliano-Caetano L. Chromosome mapping of 18S ribosomal RNA genes in eleven Hypostomus species (Siluriformes, Loricariidae): diversity analysis of the sites. Zebrafish. 2016; 13(4):360–68. https://doi.org/10.1089/zeb.2016.1279
Rubert M, Takagui FH, Santos KF, Pompeo LRS, Rosa R, Zawadzki CH et al. Topotype-based chromosomal diversity among five species of freshwater armored catfishes in the Hypostomus auroguttatus supergroup (Actinopterygii: Siluriformes). Zool Sci. 2022; 39(5):446–52. https://doi.org/10.2108/zs210103
Rubert M, Zawadzki CH, Giuliano-Caetano L. Cytogenetic characterization of Hypostomus nigromaculatus (Siluriformes, Loricariidae). Neotrop Ichthyol. 2008, 6(1):93–100. https://doi.org/10.1590/S1679-62252008000100011
Saitoh K, Miya M, Inoue JG, Ishiguro NB, Nishida M. Mitochondrial genomics of ostariophysan fishes: perspectives on phylogeny and biogeography. J Mol Evol. 2003; 56(4):464–72. https://doi.org/10.1007/s00239-002-2417-y
Santos CEV, Almeida BRR, Tavares FS, Frade LSF, Cardoso AL, Sá ALA et al. Chromosomal mapping of the histone multigene family and U2 snRNA in Hypancistrus species (Siluriformes, Loricariidae) from the Brazilian Amazon. Zebrafish. 2023; 20(1):28–36. https://doi.org/10.1089/zeb.2022.0030
Santos da Silva K, Souza ACP, Pety AM, Noronha RCR, Vicari MR, Pieczarka JC et al. Comparative cytogenetics analysis among Peckoltia species (Siluriformes, Loricariidae): insights on karyotype evolution and biogeography in the Amazon region. Front Genet. 2021; 12:779464. https://doi.org/10.3389/fgene.2021.779464
Santos da Silva K, Glugoski L, Vicari MR, Souza ACP, Noronha RCR, Pieczarka JC et al. Chromosomal diversification in Ancistrus species (Siluriformes: Loricariidae) inferred from repetitive sequence analysis. Front Genet. 2022a; 13:838462. https://doi.org/10.3389/fgene.2022.838462
Santos da Silva K, Souza ACP, Rodrigues LRR, Pieczarka JC, Nagamachi CY. Chromosomal diversification in Pseudacanthicus species (Loricariidae, Hypostominae) revealed by comparative mapping of repetitive sequences. Animals. 2022b; 12:2612. https://doi.org/10.3390/ani12192612
Santos da Silva K, Glugoski L, Vicari MR, Souza ACP, Akama A, Pieczarka JC et al. Mechanisms of karyotypic diversification in Ancistrus (Siluriformes, Loricariidae): inferences from repetitive sequence analysis. Int J Mol Sci. 2023; 24(18):14159. https://doi.org/10.3390/ijms241814159
Sassi FMC, Deon GA, Moreira-Filho O, Vicari MR, Bertollo LAC, Liehr T et al. Multiple sex chromosomes and evolutionary relationships in Amazonian catfishes: the outstanding model of the genus Harttia (Siluriformes: Loricariidae). Genes. 2020; 11(10):1179. https://doi.org/10.3390/genes11101179
Sassi FMC, Moreira-Filho O, Deon GA, Sember A, Bertollo LAC, Liehr T et al. Adding new pieces to the puzzle of karyotype evolution in Harttia (Siluriformes, Loricariidae): investigation of Amazonian species. Biology. 2021; 10(9):922. https://doi.org/10.3390/biology10090922
Sassi FMC, Sember A, Deon GA, Liehr T, Padutsch N, Oyakawa OT et al. Homeology of sex chromosomes in Amazonian Harttia armored catfishes supports the X‑fission hypothesis for the X1X2Y sex chromosome system origin. Sci Rep. 2023; 13(1):15756. https://doi.org/10.1038/s41598-023-42617-w
Schott SCQ, Glugoski L, Azambuja M, Moreira-Filho O, Vicari MR, Nogaroto V. Comparative cytogenetic and sequence analysis of u small nuclear RNA genes in three Ancistrus species (Siluriformes: Loricariidae). Zebrafish. 2022; 19(5):200–09. https://doi.org/10.1089/zeb.2022.0040
Sember A, Nguyen P, Perez MF, Altmanová M, Petr R, Cioffi MB. Multiple sex chromosomes in teleost fishes from a cytogenetic perspective: state of the art and future challenges. Philos Trans Royal Soc London B Biol Sci. 2021; 376(1833):20200098. https://doi.org/10.1098/rstb.2020.0098
Silva KS, Souza ACP, Pety AM, Noronha RCR, Vicari MR, Pieczarka JC et al. Comparative cytogenetics analysis among Peckoltia species (Siluriformes, Loricariidae): insights on karyotype evolution and biogeography in the Amazon region. Front Genet. 2021; 12:779464. http://dx.doi.org/10.3389/fgene.2021.779464
Silva M, Ribeiro ED, Matoso DA, Sousa LM, Hrbek T, Rapp Py-Daniel L et al. Chromosomal polymorphism in two species of Hypancistrus (Siluriformes: Loricariidae): an integrative approach for understanding their biodiversity. Genetica. 2014; 142(2):127–39. https://doi.org/10.1007/s10709-014-9760-y
Sochorová J, Garcia S, Gálvez F, Symnonová R, Kovarík A. Evolutionary trends in animal ribosomal DNA loci: introduction to a new online database. Chromosoma. 2018; 127:141–50. https://doi.org/10.1007/s00412-017-0651-8
Souza ACP, Nagamachi CY, Milhomem SSR, Feldberg E, Pieczarka JC. Cytogenetic analysis in catfish species of the genus Peckoltia Miranda Ribeiro, 1912 (Teleostei: Siluriformes: Loricariidae). Comp Cytogenet. 2009; 3(2):103–09. https://doi.org/10.3897/compcytogen.v3i2.17
Souza ACP, Nascimento AL, Carvalho Jr. JR, Barros RMS, Feldberg E, Nagamachi CY et al. Karyotypic analysis of Baryancistrus aff. niveatus (Ancistrinae, Loricariidae) by C-banding, AgNOR, CMA3, DAPI and FISH. Caryologia. 2004; 57(3):219–23. https://doi.org/10.1080/00087114.2004.10589396
Stace CA. Cytology and cytogenetics as a fundamental taxonomic resource for the 20th and 21st centuries. Taxon. 2000; 49:451–77. https://doi.org/10.2307/1224344
Stawikowski R, Werner A, Seidel I. Fünfzehn jahre l-nummern/fifteen years of l-numbers. Die Aquarien- und Terrarienzeitschrift Special L-Nummern/L-Numbers, 4–5. Verlag Eugen Ulmer, Stuttgart, Germany; 2004.
Sullivan JP, Lundber JG, Hardman M. A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Mol Phylogenet Evol. 2006; 41(3):636–62. https://doi.org/10.1016/j.ympev.2006.05.044
Takagui FH, Baumgärtner L, Venturelli NB, Paiz LM, Viana P, Dionísio JF et al. Unrevealing the karyotypic evolution and cytotaxonomy of armored catfishes (Loricariinae) with emphasis in Sturisoma, Loricariichthys, Loricaria, Proloricaria, Pyxiloricaria, and Rineloricaria. Zebrafish. 2020; 17(5):319–32. https://doi.org/10.1089/zeb.2020.1893
Takagui FH, Rubert M, Dionisio JF, Baumgärtner L, Cardoso YP, Jerep FC et al. Cytogenetic markers reinforce the redescription of the armored pleco Hypostomus spiniger (Loricariidae – Hypostominae), an endemic species in the Uruguay River basin and Patos Lagoon system. Braz Arch Biol Technol. 2023; 66:e23220154. https://doi.org/10.1590/1678-4324-2023220154
Takagui FH, Venturelli NB, Dias AL, Swarça AC, Vicari MR, Fenocchio AS et al. The Importance of pericentric inversions in the karyotypic diversification of the species Loricariichthys anus and Loricariichthys platymetopon. Zebrafish. 2014; 11(4):300–05. https://doi.org/10.1089/zeb.2014.0985
Traldi JB, Blanco DR, Vicari MR, Martinez JF, Lui RL, Barros AV et al. Chromosomal diversity in Hypostomus (Siluriformes, Loricariidae) with emphasis on physical mapping of 18S and 5S rDNA sites. Genet Mol Res. 2013; 12(1):463–71. http://dx.doi.org/10.4238/2013.February.8.11
Traldi JB, Lui RL, Martinez JF, Vicari MR, Nogaroto V, Moreira-Filho O et al. Chromosomal distribution of the retroelements Rex1, Rex3 and Rex6 in species of the genus Harttia and Hypostomus (Siluriformes: Loricariidae). Neotrop Ichthyol. 2019; 17(2):e190010. https://doi.org/10.1590/1982-0224-20190010
Traldi JB, Vicari MR, Blanco DR, Martinez JF, Artoni RF, Moreira-Filho O. First karyotype description of Hypostomus iheringii (Regan, 1908): a case of heterochromatic polymorphism. Comp Cytogenet. 2012; 6(2):115–25. https://doi.org/10.3897/CompCytogen.v6i2.2595
Tursellino MN, Cioffi MB, Sassi FMC, Deon GA, Oliveira C, Kuranaka M et al. Integrating genomic and chromosomal data: a cytogenetic study of Transancistrus santarosensis (Loricariidae: Hypostominae) with characterization of a ZZ/ZW sex chromosome system. Genes. 2023; 14:1662. https://doi.org/10.3390/genes14091662
Venturelli NB, Takagui FH, Pompeo LRS, Rodriguez MS, Rosa R, Giuliano-Caetano L. Cytogenetic markers to understand chromosome diversification and conflicting taxonomic issues in Rineloricaria (Loricariidae: Loricariinae) from Rio Grande do Sul coastal drainages. Biologia. 2021; 76:2561–72. https://doi.org/10.1007/s11756-021-00748-3
Ziemniczak K, Barros AV, Rosa KO, Nogaroto V, Almeida MC, Cestari MM et al. Comparative cytogenetics of Loricariidae (Actinopterygii: Siluriformes): emphasis in Neoplecostominae and Hypoptopomatinae. Italian J Zool. 2012; 79(4):492–501. https://doi.org/10.1080/11250003.2012.676677
Authors
![]() Francisco de Menezes Cavalcante Sassi1,
Francisco de Menezes Cavalcante Sassi1, ![]() Marcelo de Bello Cioffi1
Marcelo de Bello Cioffi1 ![]() and
and ![]() Orlando Moreira-Filho1
Orlando Moreira-Filho1
[1] Laboratório de Citogenética de Peixes, Departamento de Genética e Evolução, Universidade Federal de São Carlos, Rodovia Washington Luis, km 235, 13565-905 São Carlos, SP, Brazil. (FMCS) francisco.sassi@hotmail.com, (MBC) mbcioffi@ufscar.br (corresponding author), (OMF) omfilho@ufscar.br.
Authors’ Contribution 

Francisco de Menezes Cavalcante Sassi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing-original draft, Writing-review and editing.
Marcelo de Bello Cioffi: Conceptualization, Funding acquisition, Project administration, Supervision, Writing-original draft, Writing-review and editing.
Orlando Moreira-Filho: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing-review and editing.
Ethical Statement
Not applicable.
Competing Interests
The author declares no competing interests.
How to cite this article
Sassi FMC, Cioffi MB, Moreira-Filho O. A state-of-art review of Loricariidae (Ostariophysi: Siluriformes) cytogenetics. Neotrop Ichthyol. 2024; 22(4):e240050. https://doi.org/10.1590/1982-0224-2024-0050
Copyright
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Distributed under
Creative Commons CC-BY 4.0

© 2024 The Authors.
Diversity and Distributions Published by SBI
![]() Accepted June 6, 2024
Accepted June 6, 2024
![]() Submitted October 28, 2024 by Claudio Oliveira
Submitted October 28, 2024 by Claudio Oliveira
![]() Epub January 10, 2025
Epub January 10, 2025