![]() Francisco Severo-Neto1
Francisco Severo-Neto1 ![]() ,
, ![]() Karoline Ceron2,
Karoline Ceron2, ![]() Mônica Ceneviva-Bastos3,
Mônica Ceneviva-Bastos3, ![]() Alan P. Covich4 and
Alan P. Covich4 and ![]() Lilian Casatti5
Lilian Casatti5
PDF: EN XML: EN | Supplementary: S1 S2 | Cite this article
Abstract
Trophic interactions have been a long-standing field of interest in ecology, helping to understand the relationships between organisms and how ecosystems function. In this study, we describe the trophic relationships of fish from karst environments in headwater streams of the upper Paraguai River basin. We analyzed the stomach contents of 81 fish species from the Serra da Bodoquena, calculated the metrics associated with the trophic network, incorporating the body size component in the analyses, and evaluated the participation of each species in network/module connectivity. The analyzed community trophic organization was based mainly on autochthonous items which were the most consumed items for about 30% of fish species. The trophic network showed a modular pattern without nestedness or specialization. However, nestedness was significant within each module, demonstrating a hierarchical compound topology (i.e., species with few connections interacted with subsets of the pairs of more connected species within each module). We also found a relationship between network connectivity and fish body size, in which small species tend to connect modules through generalist feeding strategies. Thus, we demonstrated a still little-known role of small species in fish trophic networks, and how trophic segregation occurs in a highly diverse community from Pantanal headwater streams.
Keywords: Body size, Ecological networks, Trophic interactions, Serra da Bodoquena, Upper Paraguai.
Interações tróficas têm sido um campo de interesse de longa data na ecologia, ajudando a compreender as relações entre os organismos e como funcionam os ecossistemas. Neste estudo descrevemos as relações tróficas de peixes de ambientes cársticos em riachos de cabeceira da bacia do alto rio Paraguai. Analisamos o conteúdo estomacal de 81 espécies de peixes da Serra da Bodoquena, calculamos métricas associadas à rede trófica, incorporamos o componente de tamanho corporal nas análises e avaliamos a participação de cada espécie na conectividade da rede e módulos. A organização trófica da comunidade analisada se baseou principalmente em itens autóctones, sendo os mais consumidos por aproximadamente 30% das espécies. A rede trófica demonstrou um padrão modular, sem aninhamento ou especialização, mas com aninhamento significativo em cada módulo, demonstrando uma topologia hierárquica composta (i.e., espécies menos conectadas interagiram com subconjuntos dos parceiros de espécies mais conectadas dentro de cada módulo). Encontramos também uma relação entre a conectividade da rede e o tamanho do corpo dos peixes, em que espécies de pequeno porte tendem a conectar os módulos através de estratégias alimentares generalistas. Dessa forma, demonstramos um papel ainda pouco conhecido de espécies de pequeno porte em redes tróficas de peixes e como ocorre a segregação trófica em uma comunidade altamente diversa em riachos de cabeceira do Pantanal.
Palavras-chave: Alto Paraguai, Interações tróficas, Rede trófica, Serra da Bodoquena, Tamanho corporal.
Introduction
Fish trophic networks illustrate the intricate connections within aquatic ecosystems, delineating the flow of energy and transfer of matter among fish populations. These networks encapsulate the complex interactions between predator and prey species (Navarro et al., 2017), as well as the various trophic levels that shape ecosystem dynamics (Elliott et al., 2002). From herbivorous grazers consuming primary producers to carnivorous predators hunting smaller fish, each species occupies a unique niche within the network, contributing to its resilience and stability. Moreover, fish trophic networks reflect the interplay of environmental factors, such as habitat structure (Loch et al., 2020), nutrient availability (Winemiller, 1990), and climate (Pease et al., 2019), influencing species composition and trophic interactions. Understanding these networks is crucial for effective fisheries management and conservation efforts (Winemiller et al., 2008; Janjua, Gerdeaux, 2011) because alterations to one component can have cascading effects throughout the entire ecosystem (Su et al., 2021).
The trophic network architecture can be summarized by three community-level metrics: i) modularity (i.e., compartmentalization), a measure of species interactions within a subset of species that interacts more among themselves than with species belonging to other subset or modules; ii) nestedness, which represents the tendency of specialist-species interactions to be subsets of generalist-species interactions; and iii) specialization that designates which species restrict their interactions relative to those randomly expected based on another species availability (sensu Valdovinos, 2019). High modularity patterns in trophic networks increase network stability, retaining the impacts of disturbances in a single module and minimizing impacts over other modules (Krause et al., 2003; Teng, McCann, 2004; Grilli et al., 2016). Nested and specialized networks can minimize competition and thus increase the number of coexisting species (Bastolla et al., 2009). They are also less prone to random extinctions (Burgos et al., 2007) and habitat loss (Fortuna, Bascompte, 2006), and can be used to unravel species interaction patterns from parasites and their hosts (Campião et al., 2015) to worldwide communities (Albouy et al., 2019; Ceron et al., 2019).
In Neotropical freshwater environments, understanding fish trophic networks represents a challenge in contrast to temperate environments due to fish megadiversity, the large number of possible interactions among species, and the presence of specialized feeding niches (i.e., seed, fruit, scale, fin, and mucus feeding) (Winemiller et al., 2008). The study of fish trophic networks in Brazilian streams is still very limited (see Esteves et al., 2021 for a review) in comparison with studies in rivers and reservoirs (Uieda, Motta, 2007). However, highland streams (i.e., streams in high-altitude areas) comprise most of the worldwide freshwaters (Viviroli et al., 2003) and are characterized by high endemism. Inhabited by many specialist fishes, these streams are also important reproduction sites for many small-sized species, which make up a large portion of Neotropical fish diversity (Castro, Polaz, 2020). A high diversity of small-sized fish is a widespread pattern of species-rich animal communities (Hutchinson, MacArthur, 1959).
Karst systems, renowned for their uniqueness worldwide, originate from the dissolution of bedrocks primarily composed of carbonate limestones. This dissolution is catalyzed by the interaction of atmospheric carbon dioxide with rainfall (Hartmann et al., 2014). Karst regions cover approximately 7–12% of the Earth’s continental area, and their aquifers serve as a vital water source for nearly a quarter of the world’s population (Ford, Williams, 2007). Despite their significance, karst systems face threats from human activities, including contamination from fertilizers and pesticides, increasing water demands for agriculture, and the impacts of climate change (Hartmann et al., 2014). Karst systems in Brazil are primarily concentrated in the Northeast and Southeast regions of the country (Ferreira, Uagoda, 2020). In Central Western Brazil, this geological formation is observed in the Serra da Bodoquena region, situated on the border of the upper Paraguai basin.
There is a gap in knowledge about stream-dwelling fishes from upper Paraguai River basin in Brazil (Lima et al., 2021). Although most of this basin is composed of the Pantanal floodplain, whose hydrological dynamics are based mostly on large rivers and seasonal floods, the highlands in the eastern regions contain several small streams that drain to the main Pantanal watersheds. The southeastern upper Paraguai River basin includes the Serra da Bodoquena plateau, which consists of a 200 km length karst system where the streams drain to the Miranda and Apa River drainages, two of the major drainages of the Pantanal floodplain. Although this region has an economy based on agriculture and tourism (Velasquez et al., 2014), the streams and their riparian forests are mostly well-preserved, especially those within the Serra da Bodoquena National Park. The region is known worldwide because of the extremely high clarity of its water courses, which allows detailed observations of fishes in their natural habitat. Moreover, fish richness in these habitats is high. While the whole upper Paraguai basin contains 350 fish species (Gimênes Junior, Rech, 2022), the Serra da Bodoquena streams represent about 24% of this richness, with 83 fish species (Severo-Neto et al., 2023).
In this scenario, we aimed to characterize the diet of the Serra da Bodoquena stream fishes and to investigate how trophic interactions are structured along the consumer-resource trophic networks. We expected the highly rich community to be structured with a high degree of nestedness and specialization of interactions, which permits the coexistence of species with similar overlapping niches. Also, we expected a high degree of modularity in the community, represented by the formation of trophic groups as a response to the very nature of resource inputs to streams, with low autochthonous productivity and allochthonous input dependency.
Material and methods
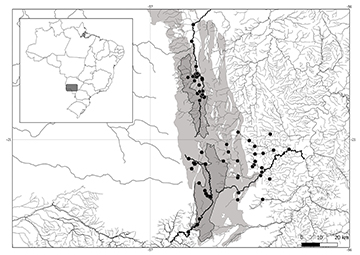
Study area and examined material. Our study was based on the fish fauna from Serra da Bodoquena, a karst plateau located in the southwestern state of Mato Grosso do Sul, Brazil (Fig. 1). With about 200 km length and up to 800 m above sea level (Sallun Filho, Karmann, 2007), the Serra da Bodoquena is in the upper Paraguai River basin and contains headwater streams that form the large rivers of the Pantanal floodplain. As a feature of karst systems, streams from the Serra da Bodoquena have high crystalline waters and high heterogeneity of substrate types, from pebbles to limestone slabs. Besides, the steep relief across the region preserves large areas of native vegetation and influences the vertical configuration of streams, which include a great diversity of meso habitats (More details in Severo-Neto et al., 2023). The Serra da Bodoquena contains the headwaters of the major Pantanal watersheds, such as the Salobra, Formoso, and Perdido rivers. The first two belong to the Miranda drainage and the latter to the Perdido drainage. The ichthyofauna of the three drainages combined is represented by 83 species, especially small nektonic fish, and includes range-restricted and endemic species (Severo-Neto et al., 2023). Material examined in this work is based on fish samples previously collected from 2003 to 2016, in first- to third-order headwater streams from 60 sites from the Serra da Bodoquena (Fig. 1) and deposited at the Coleção Zoológica da Universidade Federal de Mato Grosso do Sul, Campo Grande (ZUFMS).
FIGURE 1| Location of the fish sampling sites in Serra da Bodoquena streams, Western Brazil. The light grey area represents carbonate rocks; the Serra da Bodoquena National Park is highlighted in the dark grey shaded area.
Diet and trophic network analysis. We analyzed the stomach content of at least ten adult individuals of 83 fish species (Tab. S1). The obtained data were used to assess the feeding habitats of Serra da Bodoquena fishes, as well as the contribution of food items to the diet of each species, and the relationships of theantagonistic networks. To reduce the effect of the three basins (Salobra, Formoso, and Perdido) and to consider the whole region, fish were selected randomly among the three watersheds. Also, a rarefaction curve using the cumulative food items was conducted for each species to determine the degree of reliability of the stomach samples in the species diet. Items were identified to the lowest possible taxonomic level, as required to assemble the trophic network matrix. They were comprehensively classified as allochthonous, which included allochthonous invertebrates and plant matter, and autochthonous, including fish, scales, autochthonous invertebrates, algae, periphyton (recognizable by the presence of limestone fragments resulting from scraping action) and detritus. The contribution of each type of food to the diet of the analyzed specimen was assessed by the frequency of occurrence (%Fo), measured as the number of times that each type occurred as a percentage of the total number of occurrences of all types (Hynes, 1950). The most representative items for each species cited hereafter were those with a frequency of occurrence over 50%.
To examine the interactions between fishes and their diet, we used diet data in a complex network approach (Barabási, 2016). For this, interaction matrices A were constructed, where aij = number of interactions of a fish i with its prey j, and 0 = no interaction. We calculated metrics illustrating distinct structural properties of the network, focusing on quantitative network indices that were previously shown to be less sensitive to sampling effort (Vizentin-Bugoni et al., 2016): weighted nestedness (wNODF), modularity, and complementary specialization (H2’).
Weighted nestedness, based on the Nestedness Metric Based on Overlap and Decreasing Fill (NODF), describes the extent to which interactions of specialist species correspond to a subset of interactions of generalists (Bascompte et al., 2003). Nestedness values range from 0 (non-nested network) to 100 (perfect nesting). We also calculated modularity, whichis a network property that emerges when groups of species are densely connected and have fewer connections to other groups of interacting species (Krause et al., 2003). We analyzed modularity using the DIRTLPA algorithm (Beckett, 2016) to optimize modules based on Barber’s modularity (Barber, 2007). We set this algorithm to 100 steps to search for the highest modularity (Dormann, Strauss, 2014). Finally, we calculated the complementary specialization (H2’), which is a network-wide index of specialization for quantitative interaction matrices. It describes how species restrict their interactions from those randomly expected based on the partner’s availability (Blüthgen et al., 2006). The assumption is that if species have specific prey preferences, these preferences would be captured as a deviation from random encounters given by the partner availability (Blüthgen et al., 2006). Values of H2’ range from 0 to 1 indicating the extremes of generalization and specialization, respectively.
To assess the significance of the network patterns, we compared the observed values of wNODF, modularity, and H2’ to those generated by null models. We used the vaznull null model, which keeps the marginal totals and the connectance in the network (Dormann et al., 2008). We generated 1,000 randomized matrices to estimate nestedness and complementary specialization and 100 matrices to estimate modularity. We used fewer randomizations for modularity because their calculation is time-consuming (Olesen et al., 2007). To quantify the departure of the observed network values from the null expectation, we calculated null‐model corrected values by subtracting the observed metric value from the mean value across all randomized networks (∆ – transformation). Then, the ∆ – transformed value was divided by the standard deviation of values across all randomized networks (z – transformation; Zanata et al., 2017). We considered z values higher than two as an indication that the difference between the observed and null networks for that specific structural property was statistically significant.
After identifying the modules in the network by stipulating subsets of pairwise comparisons, we calculated nestedness between species in the same module (NSM) separately from nestedness between species in different modules (NDM) (Pinheiro et al., 2022). We also analyzed the functional role of each taxon with a cz-analysis as proposed by Guimera, Nunes Amaral (2005), and Olesen et al. (2007). This analysis calculates the coefficient of participation in the connectivity of a given species between modules (c) and within modules (z). Thresholds in c (0.62) and z (2.51) were used to classify the topological role of the species qualitatively into different ecological networks (Guimera, Nunes Amaral, 2005; Olesen et al., 2007; Borzone Mas et al., 2022). These thresholds are standardized values based on quotients of numbers of interactions and they have a specific topological meaning regardless of network size (Olesen et al., 2007). Species with both a low z and a low c were considered peripheral species or specialists, i.e., they had only a few links and were almost entirely linked to species within their module. Species with either a high value of z or c were considered generalists. These included module hubs, i.e., highly connected species linked to many species within their own module (high z, low c), and connectors linking several modules (low z, high c). Species with both a high z and a high c were defined as network hubs or super generalists, acting as both connectors and module hubs.
The association between c and z values of each species with body size (mm) was evaluated using linear models with the tendency line adjusted with a generalized linear regression in the Poisson family. Body size was represented by the mean standard length of each species in the study and was measured using a digital caliper. All network metrics and null models were calculated with the ‘bipartite’ v. 2.08 package (Dormann et al., 2008) in R software (R Development Core Team, 2022).
Results
TABLE 1 |
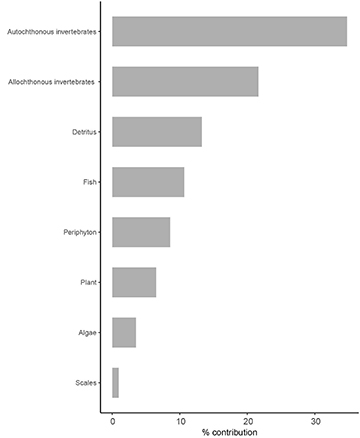
In general, Serra da Bodoquena stream fish are mostly invertivores (Fig. 2). Considering the four most frequent food types, aquatic invertebrates were the most consumed food (27%), followed by terrestrial invertebrates (21%), detritus (13%), and fish (10%). Aquatic invertebrates were represented by the larval stages of Trichoptera, Ephemeroptera, Plecoptera, Diptera, Coleoptera, Odonata, Lepidoptera, and Megaloptera, besides Mollusca and microcrustaceans, while terrestrial invertebrates were adult stages of Hymenoptera, Coleoptera, Lepidoptera, Diptera, Isoptera, Hemiptera, and Orthoptera. No items were found in the guts of Piaractus mesopotamicus and Rhyacoglanis paranensis and they were not included in the following analyses, which were done with the remaining 81 species.
FIGURE 2| Food resources and their contribution (%) to fish diet in the Serra da Bodoquena karst streams.
Aquatic invertebrates were the most representative category (above 50% of the frequency of occurrence) to 28 (~30%) fish species of the analyzed fish fauna, and Apteronotus caudimaculosus (Apteronotidae), Characidium sp.(Crenuchidae), Hyphessobrycon eques (Characidae), Imparfinis schubarti (Heptapteridae), Ituglanis herberti (Trichomycteridae) and Sternopygus macrurus (Sternopygidae) fed exclusively on invertebrates. Terrestrial invertebrates, mostly ants, contributed to the diet of 30 fish species and was the main resource for nine of them. Overall, terrestrial invertebrates predominated in the diet of Pyrrhulina australis (Lebiasinidae), Thoracocharax stellatus (Gasteropelecidae) and the Characidae Aphyocharax dentatus, Astyanax abramis, A. lacustris, A. sp. 2, Brachychalcinus retrospina, Bryconops melanurus, Hemigrammus lunatus, Moenkhausia bonita, Moenkhausia oligolepis, Phenacogaster tegatus, Piabarchus analis, Poptella paraguayensis,and Psalidodon marionae.
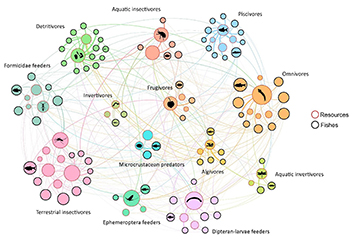
Trophic networks. The consumer-resource interaction network contained a modular pattern (modularity = 0.59, z = 22), but showed no overall nestedness or specialization (z < 2). There were 13 modules according to consumer-resource interactions (Fig. 3), with modules representing aquatic invertivores, terrestrial insectivores, frugivores, microcrustacean predators, algivores, omnivores, dipteran-larvae feeders, Formicidae feeders, invertivores, detritivores, piscivores, aquatic insectivores, and Ephemeroptera feeders. Networks within each module had significant nestedness (NSM = 22.04, p < 0.05), showing a hierarchical compound topology.
FIGURE 3| Modules recovered from fish trophic networks in Serra da Bodoquena karst streams. Circle clusters of different colors represent the trophic network modules. The size of nodes (circles) represents the degree of species/resources (i.e., the number of partners), the circles with a bold border represent fishes, and the circles with a red border indicate resources. Resource silhouettes were obtained from PhyloPic in the public domain.
We identified the species that had functional roles in structuring the network modules (Fig. 4). Most fish species were peripherals (e.g., Moenkhausia oligolepis and Brycon hilarii) or connectors (e.g., Xenurobrycon macropus and Astyanax lacustris). Resources were also categorized as peripherals (e.g., Fishes and Algae) or connectors (e.g., Coleoptera). No fishes or resources were network hubs, yet they were crucial to the coherence of both the network and its modules.
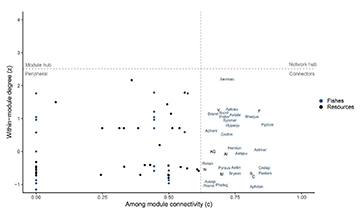
FIGURE 4| The role of species in module connectivity of fish trophic networks of Serra da Bodoquena karst streams. Species with both a low z (within module degree) and a low c (among module connectivity) are peripheral species or specialists and species with either a high value of z or c are considered generalists. Resource codes: B = Scales; C = Coleoptera; F = Ephemeroptera; M = aquatic fragments; N = Gastropoda; V = Fruits; AG = Hymenoptera; AI = adult Diptera (Tab. S2). Fish codes: Aphani – Aphyocharax anisitsi; Aphden – Aphyocharax dentatus; Aptcau – Apteronotus caudimaculosus; Astabr – Astyanax abramis; Astlin – Astyanax lineatus; Astspu – Astyanax sp. 1; Aussp – Australoheros sp.; Braret – Brachychalcinus retrospina; Bryexo – Bryconamericus exodon; Bujvit – Bujurquina vittata; Cidcim – Cichlasoma dimerus; Crelep – Saxatilia lepidota; Deulue – Deuterodon cf. luetkenii; Hemlun – Hemigrammus lunatus; Hypequ – Hyphessobrycon eques; Pheteg – Phenacogaster tegatus; Piator – Piabarchus torrenticola; Poeret – Poecilia reticulata; Psamar – Psalidodon marionae; Pseken – Psellogrammus kennedyi; Pyraus – Pyrrhulina australis; Rhaque – Rhamdia quelen; Rinlan – Rineloricaria lanceolata; Synmar – Synbranchus marmoratus; Xenmac – Xenurobrycon macropus.
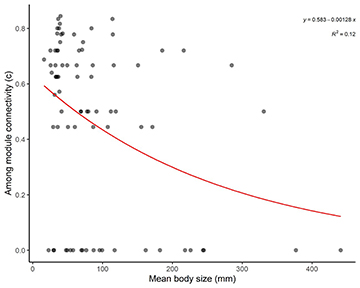
We found a relationship between connector fish species (high c value) and body size, where small-sized species tend to connect modules in the network (e.g., Aphyocharax dentatus, mean body size 35.29 mm) and larger species tend to be peripherals in the network (e.g., Hoplias malabaricus, mean body size 117.23 mm) (Fig. 5).
FIGURE 5| Relationship between module connectivity (c) and body size (mm; mean standard length) in the fish trophic network of Serra da Bodoquena karst streams.
Discussion
The diets of stream fishes in the karstic system from Serra da Bodoquena plateau were based mainly on autochthonous invertebrates, allochthonous arthropods, detritus, and fishes. Although the fish trophic network showed a non-nested and unspecialized structure, it had a modular pattern, with species aggregated in modules representing their trophic guilds. In addition, the network had a compound topology, with modules having a nested structure.
Autochthonous items are an important source of resources in streams (Saunders et al., 2018). Moreover, small streams represent unique habitats in terms of energetic balance between autotrophy and heterotrophy, determined by the predominance of autochthonous or allochthonous basal resources, respectively. Because the dense cover of riparian forests results in low autochthonous primary productivity due to the light limitation, a higher dependency on allochthonous items is expected (Vannote et al., 1980). However, recent evidence indicates that this dependence is not the only pattern to be expected in tropical streams. The high temperature and sunlight incidence in the tropics favor primary productivity (Bunn et al., 1999) and consequently an autotrophic algae-based food web (Mantel et al., 2004;Dudgeon et al., 2010;Cortés-Guzman et al., 2022), despite the level of shading provided by the riparian vegetation (Neres-Lima et al., 2016). In the Serra da Bodoquena, algae and periphyton were the main resources of 11 fish species, mostly loricariid armored catfishes. Although algivorous and periphytivorous consumers lack species richness, they represent a great part of abundance and biomass in Serra da Bodoquena streams (Froehlich, 2010). Indeed, Ancistrus sp., a small representant of armored catfishes in the region, is the second most abundant and, along with Hypostomus basilisko and H. froehlichi, adds up to almost 40% of total biomass in the Salobrinha stream (Froehlich, 2010), one of the streams covered in this work and located in the northern portion of the Serra da Bodoquena National Park.
In addition to contributing directly to the diet of grazing fish, algae and periphyton can also be important resources for macroinvertebrates which, in turn, represented the main food item to 28 species, especially those associated with feeding on the substrate, such as Characidium, Phenacorhamdia,and Pimelodella. A potential threat to this relationship comes from the trampling caused by tourists in the region, which affects the macroinvertebrate community, especially Ephemeroptera and Trichoptera (Escarpinati et al., 2014). However, such impact was not evident over small-sized insects such as Diptera larvae (Escarpinati et al., 2014), which played a major role in network modularity. Consumption of this item, especially Chironomidae, was high for “lambaris” such as Aphyocharax anisitsi, Astyanax spp., Hemigrammus lunatus, Hyphessobrycon eques, Deuterodon cf. luetkenii, Jupiaba acanthogaster, and Moenkhausia bonita. In Neotropical freshwater food webs, the role of aquatic insects, especially chironomids, is paramount both as invertebrate predators (Saigo et al., 2016) and as fish prey (Vidotto-Magnoni, Carvalho, 2009; Ceneviva-Bastos, Casatti, 2014). Most fish species from Serra da Bodoquena streams feed on aquatic insects (Fig. 2), reinforcing their role as the basis of the fish food web and a main driver of fish diversity in Neotropical streams.
Riparian forests play a key role in aquatic diversity maintenance in the Neotropics (Dala-Corte et al., 2020). The Serra da Bodoquena streams are in a diverse and considerably well-preserved riparian forest, with higher vegetation density within the conservation unit boundaries. From the structural perspective, the riparian forest provides an input of sticks and leaves that affect habitat complexity and compose the leaf litter where small fish seek refuge or food (Severo-Neto et al., 2023). In general, first- to third-order stream communities depend mostly on allochthonous sources of energy and nutrients, with a strong dependence on the terrestrial ecosystem nearby (Fausch et al., 2002; Soininen et al., 2015), and the loss or suppression of this input can alter the entire aquatic trophic web (Nakano et al., 1999; Ceneviva-Bastos, Casatti, 2014). Terrestrial arthropods that fall into stream channels represent a high-quality resource to fish (Mason, MacDonald, 1982; Edwards, Huryn, 1996; Wipfli, 1997) and its input can affect trophic cascades among insectivorous fish and periphyton biomass (Nakano et al., 1999). In Serra da Bodoquena, channel- and backwater-drift feeders depend directly on the fallen resources from the tree canopy, mostly ants, which contributed to the diet of 30 fish species and was the main resource to nine of them. Flowers, fruits, and leaves, that were included under “plant matter”, were part of the diet of 23% of the fish fauna. In terms of fish frugivory, the most emblematic species of the region is the “piraputanga” Brycon hilarii, which is known for eating fruits and dispersing seeds of several tree species (Reys et al., 2009), reinforcing the importance of large species in the seed dispersal syndrome (Galetti et al., 2008; Correa et al., 2015). However, small fish could present an important yet poorly described role in this ecological process. Fruits and seeds were found in Astyanax and Psalidodon stomachs (A. abramis, A. lacustris, A. lineatus, and P. marionae), and even the smaller Deuterodon cf. luetkenii and Jupiaba acanthogaster (ca. ~ 4 cm).
Despite the absence of a nested and specialized pattern in the karstic fish trophic network, a modular pattern was identified. The compartmentalization of networks led to the view of modules as functional units. Thus, modularity may reflect trophic guilds, habitat heterogeneity, divergent selection regimes, phylogenetic clustering of closely related species, or clusters of species converging to certain sets of traits, leading to nonrandom patterns of interaction and ultimately contributing to the complexity of ecological networks (Olesen et al., 2007). In the karstic system, the modular structure of the network can initially be related to trophic guilds of fishes in communities, i.e., the convergence of different species to the same module, and suggests trait-driven modules as identified in other studies (e.g., Donatti et al., 2011). This convergent pattern can also identify the grouping of phylogenetically close species if related species tend to have more similar dietary preferences than unrelated species (Fontaine, Thébault, 2015). This relationship was observed among reef fishes, where phylogenetic conservatism is readily apparent at the family level, leading to strongly phylogenetically structured groups (Parravicini et al., 2020). However, our results support that the modularity in these karst streams is not driven by phylogeny, because species from different families share the same modules. This lack of a taxa-related organization may reflect the unpredictability of disturbances and resource inputs in streams, leading to an overall generalism regardless of the species. Thus, the importance of modularity goes beyond the community organization, with far-reaching conservational implications for sustaining biodiversity (Olesen et al., 2007; Guimarães, 2020 ). In this sense, disturbances are expected to spread more slowly through a modular than a non-modular structure, favoring the stability of networks (Grilli et al., 2016).
We found that small-sized fish act as connectors among modules within the network, a previously unrecognized role of small fish in freshwater streams. In general, body size is a well-recognized factor affecting ecological processes (Hutchinson, MacArthur, 1959; Woodward et al., 2005; Petchey et al., 2008), from the positive relationship between trophic levels and body size (Elton, 1927; Arim et al., 2010; Reum et al., 2019; Keppeler et al., 2020), to the structuring of whole communities (Jennings et al., 2002; Romero-Romero et al., 2016). Although predators are commonly larger than their prey (Jennings et al., 2002; Barnes et al., 2010; Nakazawa, 2017) and piscivory tends to increase along the longitudinal gradient of watercourses (Oberdorff et al., 1993), the role of small fishes on trophic webs of low-order streams remains poorly known. Our results demonstrate that larger fish tend to have higher dietary specialization and, consequently, a strong relationship with the structuring of the modules. However, smaller fish species are responsible for linking the modules, acting directly on the crossed energy flow of the network.
The link between modules promoted by small fish may also be responsible for the compound topology found, where nestedness is repressed by modularity at higher levels, but prevails at lower levels, i.e., within modules (Pinheiro et al., 2022). The nested pattern within modules can be related to the tendency for specialization of larger fishes, such as the strict piscivory in Salminus brasiliensis and Pseudoplatystoma reticulatum, and the opportunistic foraging mode of small-sized species in the studied area. While small-sized species were classified as supergeneralists and connectors among modules, larger-sized species function as specialists (low z and a low c), feeding on large prey that small-sized species were unable to consume, skewing species interaction frequencies to produce highly nested configurations. Thus, this compound topology is relevant for determining if the trophic segregation of karstic communities occurs even inside structured modules. In this context, we illustrate the impact of various species with different sizes on the structure and cohesion of fish trophic networks. Larger species typically exhibit greater specialization, contributing to the maintenance of distinct modules within the network. Conversely, smaller fish species play a crucial role in connecting these modules, directly influencing the flow of energy across the network. Consequently, each species plays a significant role in upholding the structural integrity of trophic networks in Pantanal headwater streams. Nevertheless, the contribution of smaller species to fish trophic networks remains largely unexplored, despite its paramount significance in maintaining the system.
Acknowledgments
The authors are grateful for the considerations provided by Denise Rossa-Feres, Camilo Roa-Fuentes, and Maurício Cetra in the manuscript development. The authors are especially thankful for the efforts of Prof. Otávio Froehlich (in memoriam) who first initiated the works in Serra da Bodoquena that culminated in this article. KC is funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #2020/12558–0) and LC is funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq #304403/2021–0).
References
Albouy C, Archambault P, Appeltans W, Araújo MB, Beauchesne D, Cazelles K et al. The marine fish food web is globally connected. Nat Ecol Evol. 2019; 3(8):1153–61. https://doi.org/10.1038/s41559-019-0950-y
Arim M, Abades SR, Laufer G, Loureiro M, Marquet PA. Food web structure and body size: trophic position and resource acquisition. Oikos. 2010; 119(1):147–53. https://doi.org/10.1111/j.1600-0706.2009.17768.x
Barabási A-L. Network science. Cambridge: Cambridge University Press; 2016.
Barber MJ. Modularity and community detection in bipartite networks. Phys Rev E. 2007; 76:066102. https://doi.org/10.1103/PhysRevE.76.066102
Barnes C, Maxwell D, Reuman DC, Jennings S. Global patterns in predator–prey size relationships reveal size dependency of trophic transfer efficiency. Ecology. 2010; 91(1):222–32. https://doi.org/10.1890/08-2061.1
Bascompte J, Jordano P, Melián CJ, Olesen JM. The nested assembly of plant–animal mutualistic networks. Proc Natl Acad Sci USA. 2003; 100(16):9383–87. https://doi.org/10.1073/pnas.1633576100
Bastolla U, Fortuna MA, Pascual-García A, Ferrera A, Luque B, Bascompte J. The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature. 2009; 458:1018–20. https://doi.org/10.1038/nature07950
Beckett SJ. Improved community detection in weighted bipartite networks. R Soc Open Sci. 2016; 3(1):140536. https://doi.org/10.1098/rsos.140536
Blüthgen N, Menzel F, Blüthgen N. Measuring specialization in species interaction networks. BMC Ecol. 2006; 6(9). https://doi.org/10.1186/1472-6785-6-9
Borzone Mas D, Scarabotti P, Alvarenga P, Arim M. Symmetries and asymmetries in the topological roles of piscivorous fishes between occurrence networks and food webs. J Anim Ecol. 2022; 91(10):2061–73. https://doi.org/10.1111/1365-2656.13784
Bunn SE, Davies PM, Mosisch TD. Ecosystem measures of river health and their response to riparian and catchment degradation. Freshw Biol. 1999; 41(2):333–45. https://doi.org/10.1046/j.1365-2427.1999.00434.x
Burgos E, Ceva H, Perazzo RPJ, Devoto M, Medan D, Zimmermann M et al. Why nestedness in mutualistic networks? J Theor Biol. 2007; 249(2):307–13. https://doi.org/10.1016/j.jtbi.2007.07.030
Campião KM, Ribas A, Tavares LER. Diversity and patterns of interaction of an anuran–parasite network in a neotropical wetland. Parasitol. 2015; 142(14):1751–57. https://doi.org/10.1017/S0031182015001262
Castro R, Polaz CN. Small-sized fish: the largest and most threatened portion of the megadiverse neotropical freshwater fish fauna. Biota Neotrop. 2020; 20(1):e20180683. https://doi.org/10.1590/1676-0611-BN-2018-0683
Ceneviva-Bastos M, Casatti L. Shading effects on community composition and food web structure of a deforested pasture stream: evidences from a field experiment in Brazil. Limnologica. 2014; 46:9–21. http://dx.doi.org/10.1016/j.limno.2013.11.005
Ceron K, Oliveira-Santos LGR, Souza CS, Mesquita DO, Caldas FLS, Araujo AC et al. Global patterns in anuran–prey networks: structure mediated by latitude. Oikos. 2019; 128(11):1537–48. https://doi.org/10.1111/oik.06621
Cortés-Guzman D, Alcocer J, Planas D. Autotrophs are important contributors to benthic macroinvertebrate food webs in two tropical first-order forest streams. Freshw Biol. 2022; 67(6):941–53. https://doi.org/10.1111/fwb.13891
Correa SB, Araújo JK, Penha JMF, Cunha CN, Stevenson PR, Anderson JT. Overfishing disrupts an ancient mutualism between frugivorous fish and plants in Neotropical wetlands. Biol Conserv. 2015; 191:159–67. https://doi.org/10.1016/j.biocon.2015.06.019
Dala-Corte RB, Melo AS, Siqueira T, Bini LM, Martins RT, Cunico AM et al. Thresholds of freshwater biodiversity in response to riparian vegetation loss in the Neotropical region. J Appl Ecol. 2020; 57(7):1391–402. https://doi.org/10.1111/1365-2664.13657
Donatti CI, Guimarães PR, Galetti M, Pizo MA, Marquitti FMD, Dirzo R. Analysis of a hyper-diverse seed dispersal network: modularity and underlying mechanisms. Ecol Lett. 2011; 14(8):773–81. https://doi.org/10.1111/j.1461-0248.2011.01639.x
Dormann CF, Gruber B, Fründ J. Introducing the bipartite package: analysing ecological networks. R News. 2008; 8(2):8–11.
Dormann CF, Strauss R. A method for detecting modules in quantitative bipartite networks. Methods Ecol Evol. 2014; 5(1):90–98. https://doi.org/10.1111/2041-210X.12139
Dudgeon D, Cheung FKW, Mantel SK. Foodweb structure in small streams: do we need different models for the tropics? J North Am Benthol Soc. 2010; 29(2):395–412. https://doi.org/10.1899/09-058.1
Edwards ED, Huryn AD. Effect of riparian land use on contributions of terrestrial invertebrates to streams. Hydrobiologia. 1996; 337:151–59. https://doi.org/10.1007/BF00028516
Elliott M, Hemingway KL, Costello MJ, Duhamel S, Hostens K, Labropoulou M et al. Links between fish and other trophic levels. In: Michael E, Hemingway H, editors. Fishes in estuaries. Oxford: Blackwell Science Ltd. 2002; p.124–216. https://doi.org/10.1002/9780470995228.ch4
Elton CS. Animal ecology. London: Sidgewick and Jackson; 1927.
Escarpinati SC, Siqueira T, Medina-Jr PB, Roque FO. Short-term effects of visitor trampling on macroinvertebrates in karst streams in an ecotourism region. Environ Monit Assess. 2014; 186:1655–63. https://doi.org/10.1007/s10661-013-3483-x
Esteves KE, Aranha JMR, Albrecht MP. Ecologia trófica de peixes de riacho: uma releitura 20 anos depois. Oecol Aust. 2021; 25(2):282. https://doi.org/10.4257/oeco.2021.2502.04
Fausch KD, Torgersen CE, Baxter CV, Li HW. Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes: a continuous view of the river is needed to understand how processes interacting among scales set the context for stream fishes and their habitat. BioScience. 2002; 52(6):483–98. https://doi.org/10.1641/0006-3568(2002)052[0483:LTRBTG]2.0.CO;2
Ferreira CF, Uagoda RES. Um panorama sobre mapeamentos de dolinas no Brasil, feições elementares do carste. Rev Bras Geogr Fís. 2020; 13(1):302–21.
Fontaine C, Thébault E. Comparing the conservatism of ecological interactions in plant–pollinator and plant–herbivore networks. Popul Ecol. 2015; 57(1):29–36. https://doi.org/10.1007/s10144-014-0473-y
Ford D, Williams PD. Karst hydrogeology and geomorphology. New York: John Wiley & Sons; 2007.
Fortuna MA, Bascompte J. Habitat loss and the structure of plant-animal mutualistic networks. Ecology. 2006; 9(3):281–86. https://doi.org/10.1111/j.1461-0248.2005.00868.x
Froehlich O. Ictiofauna de um córrego na Serra da Bodoquena: estrutura, variações longitudinal e temporal e efeitos sobre comunidades bentônicas. [PhD Thesis]. Campo Grande: Universidade Federal de Mato Grosso do Sul; 2010. Available from: http://www.dominiopublico.gov.br/pesquisa/DetalheObraForm.do?select_action=&co_obra=198587
Galetti M, Donatti CI, Pizo MA, Giacomini HC. Big fish are the best: seed dispersal of Bactris glaucescens by the pacu fish (Piaractus mesopotamicus) in the Pantanal, Brazil. Biotropica. 2008; 40(3):386–89. https://doi.org/10.1111/j.1744-7429.2007.00378.x
Gimênes Junior H, Rech R, editors. Guia ilustrado dos peixes do Pantanal e entorno. Campo Grande: Julien Design; 2022.
Grilli J, Rogers T, Allesina S. Modularity and stability in ecological communities. Nat Commun. 2016; 7(1):12031. https://doi.org/10.1038/ncomms12031
Guimarães PR. The structure of ecological networks across levels of organization. Annu Rev Ecol Evol Syst. 2020; 51:433–60. https://doi.org/10.1146/annurev-ecolsys-012220-120819
Guimera R, Nunes Amaral LA. Functional cartography of complex metabolic networks. Nature. 2005; 433:895–900. https://doi.org/10.1038/nature03288
Hartmann A, Goldscheider N, Wagener T, Lange J, Weiler M. Karst water resources in a changing world: review of hydrological modeling approaches. Rev Geophys. 2014; 52(3):218–42. https://doi.org/10.1002/2013RG000443
Hutchinson GE, MacArthur RH. A theoretical ecological model of size distributions among species of animals. Am Nat. 1959; 93(869):117–25. https://doi.org/10.1086/282063
Hynes HBN. The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in studies of the food of fishes. J Anim Ecol. 1950; 19(1):36–58. https://doi.org/10.2307/1570
Janjua MY, Gerdeaux D. Evaluation of food web and fish dietary niches in oligotrophic Lake Annecy by gut content and stable isotope analysis. Lake Reserv Manag. 2011; 27(2):115–27. https://doi.org/10.1080/07438141.2011.566413
Jennings S, Greenstreet S, Hill L, Piet G, Pinnegar J, Warr KJ. Long-term trends in the trophic structure of the North Sea fish community: evidence from stable-isotope analysis, size-spectra and community metrics. Mar Biol. 2002; 141:1085–97. https://doi.org/10.1007/s00227-002-0905-7
Keppeler FW, Montaña CG, Winemiller KO. The relationship between trophic level and body size in fishes depends on functional traits. Ecol Monogr. 2020; 90(4):e01415. https://doi.org/10.1002/ecm.1415
Krause AE, Frank KA, Mason DM, Ulanowicz RE, Taylor WW. Compartments revealed in food-web structure. Nature. 2003; 426(6964):282–85. https://doi.org/10.1038/nature02115
Loch JMH, Walters LJ, Cook GS. Recovering trophic structure through habitat restoration: a review. Food Webs. 2020; 25:e00162. https://doi.org/10.1016/j.fooweb.2020.e00162
Lima LB, De Marco Junior P, Lima-Junior DP. Trends and gaps in studies of stream-dwelling fish in Brazil. Hydrobiologia. 2021; 848(17):3955–68. https://doi.org/10.1007/s10750-021-04616-8
Mantel SK, Salas M, Dudgeon D. Foodweb structure in a tropical Asian forest stream. J North Am Benthol Soc. 2004; 23(4):728–55. https://doi.org/10.1899/0887-3593(2004)023<0728:FSIATA>2.0.CO;2
Mason CF, MacDonald SM. The input of terrestrial invertebrates from tree canopies to a stream. Freshw Biol. 1982; 12(4):305–11. https://doi.org/10.1111/j.1365-2427.1982.tb00624.x
Nakano S, Miyasaka H, Kuhara N. Terrestrial–aquatic linkages: riparian arthropod inputs alter trophic cascades in a stream food web. Ecology. 1999; 80(7):2435–41. https://doi.org/10.1890/0012-9658(1999)080[2435:TALRAI]2.0.CO;2
Nakazawa T. Individual interaction data are required in community ecology: a conceptual review of the predator–prey mass ratio and more. Ecol Res. 2017; 32(1):5–12. https://doi.org/10.1007/s11284-016-1408-1
Navarro J, Grémillet D, Ramirez FJ, Afán I, Bouten W, Forero MG. Shifting individual habitat specialization of a successful predator living in anthropogenic landscapes. Mar Ecol Prog Ser. 2017; 578:243–51. https://doi.org/10.3354/meps12124
Neres-Lima V, Brito EF, Krsulović FAM, Detweiler AM, Hershey AE, Moulton TP. High importance of autochthonous basal food source for the food web of a Brazilian tropical stream regardless of shading. Int Rev Hydrobiol. 2016; 101(3–4):132–42. https://doi.org/10.1002/iroh.201601851
Oberdorff T, Guilbert E, Lucchetta JC. Patterns of fish species richness in the Seine River basin, France. Hydrobiologia. 1993; 259:157–67. https://doi.org/10.1007/BF00006595
Olesen JM, Bascompte J, Dupont YL, Jordano P. The modularity of pollination networks. Proc Natl Acad Sci USA. 2007; 104:19891–96. https://doi.org/10.1073/pnas.0706375104
Parravicini V, Casey JM, Schiettekatte NMD, Brandl SJ, Pozas-Schacre C, Carlot J et al. Delineating reef fish trophic guilds with global gut content data synthesis and phylogeny. PLoS Biol. 2020; 18(12):e3000702. https://doi.org/10.1371/journal.pbio.3000702
Pease AA, Capps KA, Rodiles-Hernández R, Castillo MM, Mendoza-Carranza M, Soria-Barreto M et al. Trophic structure of fish assemblages varies across a Mesoamerican river network with contrasting climate and flow conditions. Food Webs. 2019; 18:e00113. https://doi.org/10.1016/j.fooweb.2019.e00113
Petchey OL, Beckerman AP, Riede JO, Warren PH. Size, foraging, and food web structure. Proc Natl Acad Sci USA. 2008; 105(11):4191–96. https://doi.org/10.1073/pnas.0710672105
Pinheiro RB, Felix GM, Lewinsohn TM. Hierarchical compound topology uncovers complex structure of species interaction networks. J Anim Ecol. 2022; 91(11):2248–60. https://doi.org/10.1111/1365-2656.13806
R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. Available from: https://www.r-project.org/
Reum JC, Holsman KK, Aydin KY, Blanchard JL, Jennings S. Energetically relevant predator-prey body mass ratios and their relationship with predator body size. Ecol Evol. 2019; 9(1):201–11. https://doi.org/10.1002/ece3.4715
Reys P, Sabino J, Galetti M. Frugivory by the fish Brycon hilarii (Characidae) in western Brazil. Acta Oecol. 2009; 35(1):136–41. https://doi.org/10.1016/j.actao.2008.09.007
Romero-Romero S, Molina-Ramírez A, Höfer J, Acuña JL. Body size-based trophic structure of a deep marine ecosystem. Ecology. 2016; 97(1):171–81. https://doi.org/10.1890/15-0234.1
Saigo M, Marchese M, Wantzen KM. A closer look at the main actors of Neotropical floodplain food webs: functional classification and niche overlap of dominant benthic invertebrates in a floodplain lake of Paraná River. Iheringia Sér Zool. 2016; 106:e2016004. https://doi.org/10.1590/1678-4766e2016004
Sallun Filho W, Karmann I. Geomorphological map of the Serra da Bodoquena karst, west-central Brazil. J Maps. 2007; 3(1):282–95. https://doi.org/10.1080/jom.2007.9710845
Saunders WC, Bouwes N, McHugh P, Jordan CE. A network model for primary production highlights linkages between salmonid populations and autochthonous resources. Ecosphere. 2018; 9(3):e02131. https://doi.org/10.1002/ecs2.2131
Severo-Neto F, Brejão GL, Casatti L. Fish functional trophic groups in headwater karst streams from the Upper Paraguay River basin. Neotrop Ichthyol. 2023; 21(1):e220103. https://doi.org/10.1590/1982-0224-2022-0103
Soininen J, Bartels P, Heino J, Luoto M, Hillebrand H. Toward more integrated ecosystem research in aquatic and terrestrial environments. BioScience. 2015; 65(2):174–82. https://doi.org/10.1093/biosci/biu216
Su L, Liu M, You C, Guo Q, Hu Z, Yang Z et al. Nitrogen and phosphorus addition differentially enhance seed production of dominant species in a temperate steppe. Ecol. Evol. 2021; 11(21):15020–29. https://doi.org/10.1002/ece3.8185
Teng J, McCann KS. Dynamics of compartmented and reticulate food webs in relation to energetic flows. Am Nat. 2004; 164(1):85–100. https://doi.org/10.1086/421723
Uieda VS, Motta RL. Trophic organization and food web structure of southeastern Brazilian streams: a review. Acta Limnol Bras. 2007; 19(1):15–30.
Valdovinos FS. Mutualistic networks: moving closer to a predictive theory. Ecol Lett. 2019; 22(9):1517–34. https://doi.org/10.1111/ele.13279
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. The river continuum concept. Can J Fish Aquat Sci. 1980; 37(1):130–37. https://doi.org/10.1139/f80-017
Velasquez GG, Spanhol CP, Pedroso EA, Araújo EG. Stakeholders, ecotourism and sustainable development: the case of Bonito, Mato Grosso do Sul state, Brasil. Tour Hosp Int J. 2014; 2(1):133–53. https://doi.org/10.57883/thij2(1)2014.30118
Vidotto-Magnoni AP, Carvalho ED. Aquatic insects as the main food resource of fish the community in a Neotropical reservoir. Neotrop Ichthyol. 2009; 7:701–08. https://doi.org/10.1590/S1679-62252009000400020
Viviroli D, Weingartner R, Messerli B. Assessing the hydrological significance of the world’s mountains. Mt Res Dev. 2003; 23(1):32–40. https://doi.org/10.1659/0276-4741(2003)023[0032:ATHSOT]2.0.CO;2
Vizentin-Bugoni J, Maruyama PK, Debastiani VJ, Duarte LDS, Dalsgaard B, Sazima M. Influences of sampling effort on detected patterns and structuring processes of a Neotropical plant–hummingbird network. J Anim Ecol. 2016; 85(1):262–72. https://doi.org/10.1111/1365-2656.12459
Winemiller KO. Spatial and temporal variation in tropical fish trophic networks. Ecol. Monogr. 1990; 60(3):331–67. https://doi.org/10.2307/1943061
Winemiller KO, Agostinho AA, Caramaschi EP. Fish ecology in tropical streams. In: Dudgeon D, editor. Tropical stream ecology. San Diego: Elsevier/Academic Press; 2008. p.107–46. https://doi.org/10.1016/B978-012088449-0.50007-8
Wipfli MS. Terrestrial invertebrates as salmonid prey and nitrogen sources in streams: contrasting old-growth and young-growth riparian forests in southeastern Alaska, USA. Can J Fish Aquat Sci. 1997; 54(6):1259–69. https://doi.org/10.1139/f97-034
Woodward G, Ebenman B, Emmerson M, Montoya JM, Olesen JM, Valido A et al. Body size in ecological networks. Trends Ecol Evol. 2005; 20(7):402–09. https://doi.org/10.1016/j.tree.2005.04.005
Zanata TB, Dalsgaard B, Passos FC, Cotton PA, Roper JJ, Maruyama PK et al. Global patterns of interaction specialization in bird-flower networks. J Biogeogr. 2017; 44(8):1891–910. https://doi.org/10.1111/jbi.13045
Authors
![]() Francisco Severo-Neto1
Francisco Severo-Neto1 ![]() ,
, ![]() Karoline Ceron2,
Karoline Ceron2, ![]() Mônica Ceneviva-Bastos3,
Mônica Ceneviva-Bastos3, ![]() Alan P. Covich4 and
Alan P. Covich4 and ![]() Lilian Casatti5
Lilian Casatti5
[1] Coleção Zoológica da Universidade Federal de Mato Grosso do Sul, Instituto de Biociências, Rua Ufms, s/n, 79070-900 Campo Grande, MS, Brazil. netosevero@hotmail.com (corresponding author).
[2] Laboratório de Interações Ecológicas e Biodiversidade (LIEB), Departamento de Biologia, Universidade Federal do Ceará, Campus do Pici, Av. Mister Hull, s/n, 60455-760 Fortaleza, Ceará, Brazil.
[3] Departamento de Ciências Biológicas da Universidade Estadual do Centro-Oeste – Unicentro, Guarapuava, PR, Brazil. mcbastos@gmail.com.
[4] Odum School of Ecology, University of Georgia, 140 E Green Street, Athens, GA. 30602, USA. a.covich@gmail.com.
[5] Departamento de Ciências Biológicas, Universidade Estadual Paulista, Rua Cristóvão Colombo, 2265, 15054-000 São José do Rio Preto, SP, Brazil. lilian.casatti@unesp.br.
Authors’ Contribution 

Francisco Severo-Neto: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing-original draft, Writing-review and editing.
Karoline Ceron: Conceptualization, Formal analysis, Methodology, Writing-original draft, Writing-review and editing.
Mônica Ceneviva-Bastos: Conceptualization, Investigation, Validation, Writing-original draft, Writing-review and editing.
Alan P. Covich: Conceptualization, Investigation, Writing-review and editing.
Lilian Casatti: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing-original draft, Writing-review and editing.
Ethical Statement
Not applicable.
Competing Interests
The author declares no competing interests.
How to cite this article
Severo-Neto F, Ceron K, Ceneviva-Bastos M, Covich AP, Casatti L. Fish trophic network in karst streams from Brazilian Pantanal headwaters. Neotrop Ichthyol. 2024; 22(3):e240018. https://doi.org/10.1590/1982-0224-2024-0018
Copyright
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Distributed under
Creative Commons CC-BY 4.0

© 2024 The Authors.
Diversity and Distributions Published by SBI
![]() Accepted June 19, 2024 by Rosemara Fugi
Accepted June 19, 2024 by Rosemara Fugi
![]() Submitted February 21, 2024
Submitted February 21, 2024
![]() Epub August 26, 2024
Epub August 26, 2024