![]() Guilherme Moreira Dutra1
Guilherme Moreira Dutra1 ![]() ,
, ![]() Telton Pedro Anselmo Ramos2 and
Telton Pedro Anselmo Ramos2 and ![]() Naércio Aquino Menezes1
Naércio Aquino Menezes1
PDF: EN XML: EN | Cite this article
Abstract
Three new species of Eigenmannia belonging to the E. trilineata species-group are described. The first species is described from rio Mearim basin and can be diagnosed by lateral line stripe restricted to last two thirds of body, superior midlateral stripe present, 176–205 anal-fin rays, 10–15 scales rows above lateral line, 109–125 lateral line scales, 19–23 premaxillary teeth, 20–29 dentary teeth, 6–10 endopterygoid teeth, and 13–14 precaudal vertebrae. The second species is described from upper rio Parnaíba, and can be diagnosed by lateral line stripe restricted to last two-thirds of body, ii,11–13 pectoral-fin rays, 180–196 anal-fin rays, 12–15 scales rows above lateral line, 10–14 premaxillary teeth, 15–21 dentary teeth, 8–10 endopterygoid teeth, and 14 precaudal vertebrae. The third species is widespread in rio Parnaíba basin, and can be diagnosed by absence of lateral line stripe, absence of superior midlateral stripe, 182–228 anal-fin rays, 12–15 scales rows above lateral line, 107–131 lateral line scales, 32–34 premaxillary teeth, 35–44 dentary teeth, 9–12 endopterygoid teeth, and 13 precaudal vertebrae. A dichotomous key and the conservation status for the three species are provided.
Keywords: Electric fishes, Eigenmannia trilineata species-group, Identification key, Taxonomy.
Três espécies novas de Eigenmannia pertencentes ao grupo E. trilineata são descritas. A primeira espécie é descrita para a bacia do rio Mearim e pode ser diagnosticada por apresentar faixa da linha lateral restrita aos últimos dois terços do corpo, faixa médio lateral superior presente, 176–205 raios na nadadeira anal, 10–15 fileiras de escamas acima da linha lateral, 109–125 escamas na linha lateral, 19–23 dentes pré-maxilares, 20–29 dentes no dentário, 6–10 dentes no endopterigóide, e 13–14 vértebras pré-caudais. A segunda espécie é descrita do alto rio Parnaíba, e pode ser diagnosticada por apresentar faixa da linha lateral restrita aos últimos dois terços do corpo, ii,11–13 raios na nadadeira peitoral, 180–196 raios na nadadeira anal, 12–15 fileiras de escamas acima da linha lateral, 10–14 dentes pré-maxilares, 15–21 dentes no dentário, 8–10 dentes no endopterigóide, e 14 vértebras pré-caudais. A terceira espécie está amplamente distribuída na bacia do rio Parnaíba, e pode ser diagnosticada pela ausência de faixa na linha lateral, ausência da faixa médio lateral superior, 182–228 raios na nadadeira anal, 12–15 fileiras de escamas acima da linha lateral, 107–131 escamas na linha lateral, 32–34 dentes pré-maxilares, 35–44 dentes no dentário, 9–12 dentes no endopterigóides, e 13 vértebras pré-caudais. Uma chave dicotômica e o status de conservação para as três espécies são fornecidas.
Palavras-chave: Chave de identificação, Peixes elétricos, grupo de espécies Eigenmannia trilineata, Taxonomia.
Introduction
Eigenmannia Jordan & Evermann is the most species-rich genus of the Sternopygidae, comprising 27 valid species (Ferraris et al, 2017; Campos-da-Paz, Queiroz, 2017; Dutra et al., 2017, 2018, 2021; Peixoto, Waltz, 2017; Waltz, Albert, 2018; Peixoto, Ohara, 2019; Herrera-Collazos et al., 2020; Peixoto et al., 2021). It includes small to medium-sized fishes (120–490 mm TL) that are widely distributed throughout the Neotropics, from the Río Tuíra basin in Panamá to the Río de La Plata basin in Argentina (Waltz, Albert, 2017). The monophyly of Eigenmannia was recently demonstrated by Alda et al. (2019) based on the analysis of ultra-conserved genetic elements and ratified by Dutra et al. (2021) on the basis of four morphological synapomorphies: (1) presence of “epipleurals” at 7–9th vertebrae, (2) Nervus opticus thicker than Nervus olfactorius, (3) number of premaxillary teeth (30–37), and (4) five premaxillary teeth rows.
In the Parnaíba ecoregion (sensu Abell et al., 2008), the first record of Eigenmannia was made by Fowler (1941), who suggested the presence of E. virescens (Valenciennes, 1836) in the rio Parnaíba at Teresina. Subsequently, E. virescens was reported in the same basin by Ramos et al. (2014), and in its tributaries rio Gurgueia (Silva et al., 2015), and rio Longá (Melo et al., 2016). Likewise, E. virescens has also been reported in the rio Itapecuru and rio Pindaré, tributaries of Mearim basin (Piorski, 1998; Guimarães et al., 2020), another relatively large drainage of northeastern Brazil. However, Peixoto et al. (2015) argue that the distribution of E. virescens is restricted to the lower portions of the rio Paraná basin and the Río la Plata. Consequently, there is a clear necessity to reevaluate the identity of Eigenmannia populations in the rio Mearim and rio Parnaíba basins. Thus, the aim of this study is to conduct a comprehensive taxonomic revision of Eigenmannia in these basins.
Material and methods
All measurements were taken point-to-point to the nearest 0.1 mm with digital calipers under a stereomicroscope, preferably on the left side. Measurements and counts followed Peixoto et al. (2015). Measurements reported as a percentage of total length and length to end of anal-fin base are given only for specimens without damage or regeneration. In the description, frequencies are given in parentheses after each count and an asterisk indicates counts for the holotype. Osteological data were obtained via specimens that were cleared and stained using the technique of Taylor, Van Dyke (1985). Precaudal vertebrae includes four vertebrae of the Weberian complex plus all remaining vertebrae without fully developed hemal spines. Transitional vertebrae are post-Weberian precaudal vertebrae lacking both pleural ribs and hemal spines (Hopkins, 1991). The nomenclature of stripes follows Peixoto et al. (2015) and Peixoto, Wosiacki (2016) (Fig. 1). Information from literature was also used for comparisons (Waltz, Albert, 2018; Herrera-Collazos et al., 2020). Abbreviations used in the text are: CS = cleared and counterstained, HL = head length, and LEA = length to the end of the anal fin. Institutional abbreviations follow Sabaj (2019).
FIGURE 1| Schematic showing the body stripe nomenclature of Peixoto et al. (2015) and Peixoto, Wosiacki (2016).
Results
Eigenmannia bumba, new species
urn:lsid:zoobank.org:act:3F3C0B90-6FB3-43E8-9B03-A158FD487271
(Figs. 2, 3A, B; Tab. 1)
Eigenmannia virescens (non Valenciennes, 1836). — Guimarães et al., 2020:7 (listed, ichthyofauna of the Pindaré River).
Holotype. MZUSP 125870, 117.5 mm LEA, rio Santana, tributary of rio Grajaú, rio Mearim basin, Grajaú, Maranhão, Brazil, 05°35’39.48”S 46°14’30.88”W, O. Oyakawa, F. Dagosta, M. Marinho & P. Camelier, 16 Out 2014.
Paratypes. MZUSP 123709, 22+3CS, 77.5–123.5 mm LEA; MZUSP 123714, 21, 53.6–107.1 mm LEA, collected with holotype.
Non-types. MZUSP 5065, 1, 89.7 mm LEA, Rio Grajaú, Grajaú, Maranhão, 5°49”S 46°09’W, Expedição do Departamento de Zoologia, 15 Jun 1966. MZUSP 125873, 1, 83.9 mm LEA, Rio Pindaré, Bom Jesus das Selvas, Maranhão, 4°36’32.6”S 46°56’09.2”W, O. Oyakawa, F. Dagosta, M. Marinho, P. Camelier, 21 Out 2014.
Diagnosis. Eigenmannia bumba, a member of the E. trilineata species-group, differs from the E. humboldtii species-group by the anal-fin hyaline (vs. anal-fin margin distinctly darkened), and from E. macrops (Boulenger, 1897) by the absence of enlarged eye (21.3–25.4% HL vs. 26.4–29.7% HL), and the presence of a short caudal filament (19.2–30.8% LEA vs. 67.5–79.3% LEA). Within the E. trilineata species-group,the new species differs from all other species, except E. besouro Peixoto & Wosiacki, 2016, E. correntes Campos-da-Paz & Queiroz, 2017, E. dutrai Peixoto, Pastana & Ballen, 2021, E. guchereauae (Meunier, Jegu & Keith, 2014), E. meeki Dutra, de Santana & Wosiacki, 2017, E. oradens Dutra, Peixoto, de Santana & Wosiacki, 2018, E. robsoni, E. sirius Peixoto & Ohara, 2019, E. vicentespeleaea Triques, 1996, E. virescens, and E. waiwai Peixoto, Dutra & Wosiacki, 2015 by having a subterminal mouth (vs. terminal). Eigenmannia bumba differs from the aforementioned species by the following combination of characters: (1) lateral line stripe restricted to last two thirds of body (vs. complete in E. besouro, E. correntes, E. dutrai, E. guchereauae, E. meeki, E. oradens, E. sirius, E. vicentespelaea, and E. waiwai); (2) superior midlateral stripe present (vs. absent in E. guchereauae E meeki, E. oradens, E. robsoni, and E. virescens); (3) 176–205 anal-fin rays (vs. 143–154 in E. correntes, 211–204 in E. meeki); (4) 10–15 scales rows above lateral line (vs. 7–8in E. vicentespelaea); (5) 109–125 scales on lateral line (vs. 140–168 in E. meeki), (6) 19–23 premaxillary teeth (vs. 75 in E. guchereauae, 30–55 in E. meeki, 38–42 in E. oradens, 32–34 in E. robsoni,25–26 in E. vicentespeleaea, and 35–40 in E. waiwai); (7) 20–29 dentary teeth (vs. 16–18 in E. correntes, 35–36 in E. dutrai, 88 in E. guchereauae, 31–38 in E. oradens, 35–44 in E. robsoni,38–41 in E. vicentespelaea, 39 in E. virescens, and 37–38 in E. waiwai); (8) 6–10 endopterygoid teeth (vs. 13–15 in E. meeki,14–17 in E. waiwai); (9) depth of posterodorsal expansion on infraorbitals 1+2 half as long as infraorbitals 1+2 length (vs. as long as infraorbitals 1+2 length in E. dutrai, E. guchereauae, E. oradens, E. sirius); (10) basibranchial 1 unossified (vs. ossified in E. virescens); (11) 13–14 precaudal vertebrae (vs. 15 in E. meeki and E. sirius); (12) length of coronomeckelian bone corresponding to 20% of Meckel’s cartilage length (vs. 45% in E. oradens and E. waiwai). A summary of diagnostic characters among species of the E. trilineata species-group is provided on Tabs. 2–3.
Description. Body shape and pigmentation shown in Fig. 2, morphometric data in Tab. 1. Largest examined specimen 123.5 mm LEA. Body elongate and distinctly compressed. Greatest body depth at vertical crossing distal tip of pectoral fin. Dorsal profile of body slightly convex from snout tip to vertical through anal-fin terminus. Ventral profile of body convex from tip of lower jaw to anal-fin terminus. Caudal filament short.
FIGURE 2| Eigenmannia bumba, MZUSP 125870, holotype, 117.5 mm LEA, rio Santana, Grajaú, Maranhão, Brazil, 05°35’39.48”S 46°14’30.88”W. A. lateral view of head, B. lateral view of body.
TABLE 1 | Morphometrics for examined specimens of Eigenmannia bumba, E. cacuria, and E. robsoni.
Eigenmannia bumba | Eigenmannia cacuria | Eigenmannia robsoni | ||||||||||||||||
Holotype | Min | Max | Mean | SD | N | Holotype | Min | Max | Mean | SD | N | Holotype | Min | Max | Mean | SD | N | |
Total length (mm) | 117.5 | 104.8 | 148.7 | – | – | 15 | 152.4 | 74.9 | 152.4 | – | – | 7 | 175.1 | 83.8 | 151.8 | – | – | 19 |
Length to end of anal fin (mm) | 148.7 | 83.5 | 123.5 | – | – | 15 | 110.0 | 53.6 | 134.0 | – | – | 10 | 139.3 | 110.5 | 207.0 | – | – | 14 |
Head length (mm) | 14.0 | 11.3 | 16.5 | – | – | 15 | 12.9 | 7.5 | 14.3 | – | – | 10 | 16.1 | 12.1 | 19.0 | – | – | 19 |
Caudal-filament length (mm) | 31.2 | 17.0 | 31.2 | – | – | 15 | 42.5 | 21.3 | 42.5 | – | – | 7 | 35.8 | 21.9 | 55.2 | – | – | 14 |
Percent
of length to the end of anal fin | ||||||||||||||||||
Caudal-filament length | 26.6 | 19.2 | 30.8 | 24.7 | 3.4 | 15 | 38.6 | 26.4 | 41.3 | 36.7 | 4.9 | 7 | 25.7 | 19.9 | 36.4 | 27.2 | 5.0 | 14 |
Greatest body depth | 17.7 | 15.5 | 17.7 | 16.4 | 0.7 | 15 | 16.1 | 14.8 | 17.8 | 16.6 | 0.8 | 9 | 13.9 | 13.5 | 18.7 | 15.6 | 1.7 | 17 |
Body depth at anal-fin origin | 15.3 | 13.2 | 15.5 | 14.4 | 0.8 | 15 | 14.7 | 12.9 | 16.2 | 14.9 | 0.9 | 9 | 12.8 | 12.8 | 18.5 | 14.5 | 1.6 | 18 |
Body width | 7.2 | 5.4 | 7.6 | 6.4 | 0.6 | 15 | 6.2 | 5.3 | 7.6 | 6.5 | 0.6 | 9 | 5.6 | 4.9 | 8.5 | 6.0 | 0.9 | 18 |
Preanal-fin distance | 17.1 | 15.4 | 18.9 | 17.6 | 0.9 | 15 | 16.3 | 13.3 | 19.1 | 17.1 | 1.7 | 9 | 14.7 | 14.7 | 20.2 | 17.1 | 1.8 | 18 |
Prepectoral-fin distance | 13.2 | 10.1 | 15.8 | 13.8 | 1.6 | 15 | 12.5 | 11.2 | 15.0 | 13.7 | 1.2 | 9 | 12.8 | 10.2 | 16.5 | 13.9 | 1.5 | 18 |
Anal-fin length | 85.4 | 78.1 | 89.4 | 84.8 | 2.8 | 15 | 86.4 | 82.5 | 88.3 | 86.0 | 1.5 | 9 | 87.5 | 81.3 | 89.9 | 85.0 | 2.6 | 18 |
Pectoral-fin length | 8.4 | 8.4 | 10.6 | 9.8 | 0.6 | 15 | 8.2 | 7.2 | 10.3 | 8.6 | 1.1 | 9 | 8.8 | 0.0 | 12.2 | 9.5 | 2.6 | 18 |
Snout to anus | 7.6 | 7.6 | 12.2 | 9.7 | 1.3 | 15 | 7.6 | 6.0 | 13.6 | 10.1 | 2.7 | 9 | 7.5 | 6.9 | 12.1 | 8.9 | 1.5 | 17 |
Head length | 11.9 | 11.9 | 14.1 | 13.2 | 0.7 | 15 | 11.7 | 10.7 | 14.0 | 12.9 | 1.1 | 9 | 11.5 | 11.5 | 15.0 | 13.1 | 1.1 | 18 |
Percent
of head length | ||||||||||||||||||
Head width at opercle | 59.2 | 52.6 | 59.3 | 57.0 | 1.9 | 15 | 59.5 | 54.8 | 64.7 | 58.8 | 3.1 | 10 | 55.7 | 51.0 | 61.4 | 55.1 | 3.0 | 19 |
Head width at eye | 52.2 | 40.6 | 52.2 | 48.2 | 3.0 | 15 | 50.0 | 41.6 | 52.5 | 47.9 | 3.6 | 10 | 47.0 | 42.1 | 49.8 | 45.4 | 2.1 | 19 |
Head depth at nape | 81.0 | 76.1 | 83.3 | 79.5 | 2.2 | 15 | 86.5 | 81.4 | 89.6 | 85.5 | 3.0 | 10 | 81.6 | 73.3 | 88.0 | 79.3 | 3.9 | 19 |
Head depth at eye | 59.7 | 57.6 | 66.5 | 61.8 | 2.5 | 15 | 69.2 | 58.6 | 69.2 | 65.1 | 3.3 | 10 | 62.6 | 54.9 | 66.2 | 60.2 | 3.0 | 19 |
Snout length | 32.4 | 31.7 | 37.0 | 33.7 | 1.3 | 15 | 35.3 | 29.1 | 35.3 | 32.2 | 1.7 | 10 | 37.2 | 30.5 | 37.2 | 33.7 | 1.9 | 19 |
Snout to posterior nostril | 24.7 | 21.1 | 24.7 | 22.9 | 1.2 | 15 | 23.2 | 20.8 | 24.8 | 22.8 | 1.1 | 10 | 25.1 | 20.3 | 26.0 | 23.6 | 1.5 | 19 |
Posterior nostril to eye | 10.6 | 8.3 | 11.6 | 9.9 | 0.9 | 15 | 11.3 | 8.5 | 12.7 | 10.7 | 1.2 | 10 | 10.4 | 8.4 | 23.9 | 10.7 | 3.5 | 19 |
Postorbital distance | 48.1 | 48.0 | 53.3 | 50.0 | 1.7 | 15 | 54.8 | 49.8 | 54.9 | 52.6 | 1.8 | 10 | 55.2 | 48.2 | 57.8 | 52.1 | 2.7 | 19 |
Branchial opening | 31.6 | 26.8 | 36.9 | 30.6 | 2.7 | 15 | 30.5 | 27.9 | 33.6 | 30.7 | 1.8 | 10 | 27.4 | 24.7 | 32.9 | 30.4 | 2.2 | 19 |
Internarial width | 19.2 | 16.8 | 23.4 | 20.0 | 1.7 | 15 | 19.7 | 17.2 | 21.0 | 19.9 | 1.1 | 10 | 21.4 | 17.4 | 21.5 | 19.9 | 1.1 | 19 |
Internarial distance | 12.5 | 10.5 | 13.5 | 11.9 | 0.9 | 15 | 12.0 | 10.5 | 13.3 | 11.9 | 0.9 | 10 | 11.1 | 9.3 | 14.1 | 11.7 | 1.1 | 19 |
Interorbital distance | 34.9 | 31.0 | 36.6 | 33.1 | 1.5 | 15 | 41.6 | 34.3 | 43.1 | 39.0 | 2.5 | 10 | 33.4 | 29.1 | 36.4 | 32.1 | 1.9 | 19 |
Eye diameter | 21.6 | 21.3 | 25.4 | 23.8 | 1.3 | 15 | 17.5 | 17.5 | 21.1 | 19.0 | 1.1 | 10 | 18.1 | 17.2 | 24.5 | 20.6 | 1.9 | 19 |
Mouth length | 19.3 | 17.1 | 23.6 | 19.3 | 1.8 | 15 | 23.4 | 18.4 | 23.5 | 21.1 | 1.9 | 10 | 19.0 | 17.1 | 23.6 | 20.2 | 1.6 | 19 |
Mouth width | 18.7 | 16.6 | 20.3 | 18.4 | 1.1 | 15 | 18.8 | 14.5 | 22.2 | 19.2 | 2.1 | 10 | 17.6 | 16.2 | 21.3 | 18.1 | 1.3 | 19 |
Percent
of caudal filament length | ||||||||||||||||||
Caudal-filament width | 3.6 | 3.0 | 4.4 | 3.7 | 0.4 | 9 | 2.7 | 1.8 | 3.5 | 2.8 | 0.6 | 7 | 4.2 | 2.2 | 7.7 | 3.9 | 1.6 | 12 |
Caudal-filament depth | 6.1 | 4.8 | 10.2 | 6.8 | 1.6 | 9 | 3.6 | 3.3 | 6.5 | 4.3 | 1.1 | 7 | 8.4 | 4.7 | 10.2 | 6.9 | 1.6 | 12 |
TABLE 2 | Summary of external and osteological diagnostic characters for the 26 recognized species of the Eigenmannia trilineata species-group. Table redrawn and modified from Waltz, Albert (2018) based on compiled data from Peixoto et al. (2015), Peixoto, Wosiacki (2016), Campos-da-Paz, Queiroz (2017), Dutra et al. (2017, 2018, 2021), Peixoto, Waltz (2017), Peixoto, Ohara (2019), Herrera-Collazos et al. (2020), and Peixoto et al. (2020). Dashes represent data not available in the literature.
Mouth position | Lateral line stripe | Superior midlateral stripe | Size of teeth along dentigerous surface of dentary | Depth of posterodorsal expansion on infraorbitals
1+2 (IO 1+2) | Coronomeckelian bone length | Basibranchial 1 | |
E. antonioi | terminal | present | present | increasing in size | as long as IO 1+2
length | 20% MC | unossified |
E. besouro | subterminal | present | present | all similar in size | 40% as long as IO
1+2 length | 30% MC | unossified |
E. bumba | subterminal | present | present | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. cacuria | terminal | present | present | increasing in size | as long as IO 1+2
length | 20% MC | unossified |
E. camposi | terminal | present | – | – | – | – | – |
E. correntes | subterminal | present | present | increasing in size | 40% as long as IO
1+2 length | 20% MC | unossified |
E. desantanai | terminal | present | present | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. dutrai | subterminal | present | present | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. guairaca | terminal | present | present | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. guchereauae | subterminal | present | absent | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. loretana | terminal | present | present | – | 60–75% as long as IO
1+2 length | 20% MC | – |
E. magoi | terminal | present | present | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. matintapereira | terminal | present | present | all similar in size | half as long as IO
1+2 length | 20% MC | unossified |
E. meeki | subterminal | present | absent | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. microstoma | terminal | present | present | all similar in size | as long as IO 1+2
length | 45% MC | unossified |
E. muirapinima | terminal | present | present | increasing in size | as long as IO 1+2
length | 20% MC | unossified |
E. oradens | subterminal | present | absent | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. pavulagem | terminal | present | present | increasing in size | as long as IO 1+2
length | 20% MC | unossified |
E. robsoni | subterminal | absent | absent | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. sayona | terminal | present | present | increasing in size | as long as IO 1+2
length | 20% MC | ossified |
E. sirius | subterminal | present | present | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. trilineata | terminal | present | present | all similar in size | half as long as IO
1+2 length | 20% MC | unossified |
E. vicentespelaea | subterminal | present | present | all similar in size | as long as IO 1+2
length | 20% MC | unossified |
E. virescens | subterminal | absent | absent | all similar in size | half as long as IO
1+2 length | 20% MC | ossified |
E. waiwai | subterminal | present | present | all similar in size | half as long as IO
1+2 length | 20% MC | unossified |
E. zenuensis | terminal | present | present | – | – | – | – |
TABLE 3 | Summary of meristic diagnostic characters for the 26 recognized species of the Eigenmannia trilineata species-group. Table redrawn and modified from Waltz, Albert (2018) based on compiled data from Peixoto et al. (2015), Peixoto, Wosiacki (2016), Campos-da-Paz, Queiroz (2017), Dutra et al. (2017, 2018, 2021), Peixoto, Waltz (2017), Peixoto, Ohara (2019), Herrera-Collazos et al. (2020), and Peixoto et al. (2021).
Pectoral-fin
rays | Anal-fin
rays | Scale
rows above lateral line | Premaxillary
teeth | Dentary
teeth | Endopterygoid teeth | Precaudal
vertebrae | |
E. antonioi | ii,13–14 | 166–207 | 8–10 | 8–12 | 8–15 | 8–9 | 13–14 |
E. besouro | ii,12–15 | 150–198 | 7–10 | 18–29 | 19–30 | 10–11 | 14 |
E. bumba | ii,13–15 | 176–205 | 10–15 | 19–23 | 20–29 | 6–10 | 13–14 |
E. cacuria | ii,11–13 | 180–196 | 12–15 | 10 | 15–21 | 8–10 | 14 |
E. camposi | ii,12–15 | 173–217 | 7–10 | 27 | 20–22 | 9–10 | 13 |
E. correntes | ii,12–13 | 143–154 | 11–12 | 11–20 | 16–18 | 4–9 | 14 |
E. desantanai | ii,12–14 | 170–198 | 8–10 | 24–25 | 21–23 | 14–15 | 11–12 |
E. dutrai | ii,14–16 | 171–206 | 9–11 | 23–29 | 35–36 | 9–18 | 14–16 |
E. guairaca | ii,11–12 | 151–170 | 14–15 | 9–10 | 17–19 | 5–6 | 15 |
E. guchereauae | ii,16–19 | 184–187 | 8–12 | 75 | 88 | ? | 13–14 |
E. loretana | ii,13–14 | 162–196 | 9–13 | 11–15 | 17–19 | 6–7 | 13–14 |
E. magoi | ii,11–16 | 182–259 | 6–18 | 32 | 35–39 | 11 | 14 |
E. matintapereira | ii,16–17 | 216–222 | 10–12 | 22–24 | 25–27 | 9–12 | 13 |
E. meeki | ii,13–17 | 211–240 | 10–15 | 30–35 | 21–23 | 13–15 | 15 |
E. microstoma | ii,12–15 | 173–207 | 11–15 | 16 | 16 | 11–16 | 14–16 |
E. muirapinima | ii,11–12 | 170–198 | 8–13 | 8–10 | 11–16 | 8–9 | 13–14 |
E. oradens | ii,16–17 | 164–192 | 8–11 | 38–42 | 31–38 | 10 | 14 |
E. pavulagem | ii,13–14 | 176–201 | 8–12 | 13–16 | 15–21 | 8–11 | 13–15 |
E. robsoni | ii,12–15 | 182–228 | 12–15 | 25–30 | 35–40 | 9–12 | 13 |
E. sayona | ii,12–13 | 198–217 | 9–11 | 17 | 19–26 | 8–9 | 13 |
E. sirius | ii,13–15 | 157–183 | 9–12 | 15–24 | 15–33 | 9–13 | 15 |
E. trilineata | ii,14–15 | 176–217 | 9–12 | 31–33 | 23 | 16–17 | 14 |
E. vicentespelaea | ii,15–17 | 169–225 | 7–8 | 25–26 | 38–41 | 10–5 | 13–14 |
E. virescens | ii,16–17 | 173–225 | 9–11 | 22 | 39 | 9 | 14 |
E. waiwai | ii,12–16 | 167–195 | 9–10 | 35–40 | 37–38 | 14–17 | 12–13 |
E. zenuensis | ii,12–16 | 180–222 | 7–9 | 31–34 | 56–60 | 7–11 | 14–15 |
Head laterally compressed; greatest width at opercular region, greatest depth at nape. Dorsal profile of head convex from snout tip to nape. Ventral profile of head convex from tip of lower jaw to isthmus. Snout pointed in lateral view. Mouth subterminal. Mouth rictus at vertical through a point between anterior and posterior nares or vertical through posterior nostril. Anterior nostril tube-like; closer to snout tip than to anterior margin of eye. Posterior nostril round, not tubular, closer to anterior margin of eye than to snout tip, at horizontal line between middle and dorsal margin of eye. Eye small, circular, completely covered by skin, on anterior one-half of HL, laterally oriented. Anus adjacent to urogenital papilla, shifting ontogenetically from vertical through middle of opercle to vertical through posterior margin of eye. Urogenital papilla usually not developed in specimens under 95.5 mm LEA. Branchial membranes joined at isthmus. Gill rakers on first branchial arch 11(3).
Scales cycloid, small, extending from posterior most part of head to vertical through tip of caudal-filament, present on mid-dorsal region of body. Scales above lateral line at vertical through end of pectoral fin 12(1), 13(6), 14*(4), or 15(4). Anterior most perforated lateral-line scale along vertical through pectoral-fin origin. Lateral-line scales to vertical through base of last anal-fin ray 109–125 (N = 15), 119 in holotype.
Pectoral-fin rays ii,13*(2), ii,14(10), or ii,15(3). Distal pectoral-fin margin straight. Total anal-fin rays 176–205(N = 15), 202 in holotype. Anal-fin origin along vertical through pectoral-fin insertion or slightly posterior. Distal margin of anal fin slightly convex. First unbranched rays tiny, subsequent rays progressively increasing in size toward first branched rays. Branched rays of nearly equal length except for posterior most rays that progressively decrease in length.
Relevant osteological features. Premaxillary teeth 19(1) or 23(2) in four(3) rows (Fig. 3A). Dentary teeth 20(1), 26(1), or 29(1) in two(2) or three(1) rows (Fig. 3B). Dentary teeth not increasing in size along dentigerous surface. Coronomeckelian bone length near 20% Meckel’s cartilage length. Endopterygoid teeth six (1), seven (1), or 10 (1) in one (2) or two (1) rows. Antorbital and infraorbitals 1 to 4 enlarged, partially cylindrical with slender osseous arches. Fifth and sixth infraorbitals slender and tubular. Depth of posterodorsal expansion on infraorbitals 1+2 half as long as infraorbitals 1+2 length. Branchiostegals five(3). Upper pharyngeal teeth six(1), seven(1), or eight(1). Lower pharyngeal teeth nine(1), 10(1) or 13(1). Precaudal vertebrae 13(2), or 14(1). Transitional vertebrae four(3). Pleural ribs six(2) or seven(1). Displaced hemal spines three(3).
FIGURE 3| Osteological elements of Eigenmannia bumba, MZUSP 123709: A. Premaxillae, B. Lower jaw; Eigenmannia cacuria, UFPB 11916: C. Premaxillae, D. Lower jaw; and Eigenmannia robsoni,UFPB 8202: E. Premaxillae, F. Lower jaw.
Coloration in alcohol. Body ground coloration cream. Body with two layers of chromatophores. Outer layer covered by dark chromatophores gradually more spaced ventrally, more concentrated between lateral line and anal pterygiophores forming a superior midlateral stripe. Lateral line stripe faint and restricted to last two thirds of body. Superior medial stripe thick, covering the space equivalent to one to three scales vertically, tapering from vertical through end of body cavity to vertical through to posterior one-third of LEA. Inner layer of pigmentation formed by multiple, small bars of dark chromatophores situated between the musculature associated with anal-fin pterygiophores. Dark individual bars in combination forming a stripe-like pattern on anal-fin base. Anal-fin base stripe approximately half as wide as orbital diameter. Head covered by dark chromatophores, more concentrated on opercular region. Pectoral and anal fins hyaline with scattered dark chromatophores overlying fin rays.
Geographical distribution. Eigenmannia bumba is known from rio Grajaú and rio Pindaré, both tributaries of the rio Mearim, Northeastern Brazil (Fig. 4).
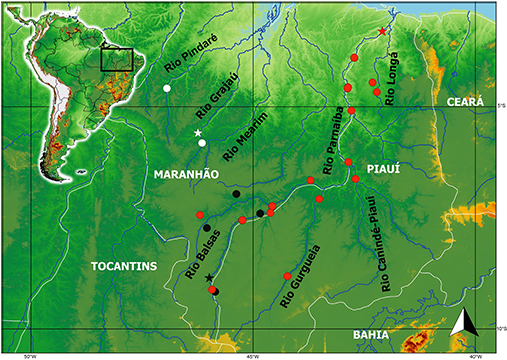
FIGURE 4| Map of Northern Brazil showing distribution of Eigenmannia bumba (white), Eigenmannia cacuria (black), and E. robsoni (red). A star indicates the type locality.
Ecological notes. Specimens of Eigenmannia bumba were collected in clear and slow flowing waters with substrates comprising sand and organic matter. Sampled sites were 5 to 15m in width and 1.7m deep, with riparian vegetation dominated by grassy and herbaceous vegetation.
Etymology. The epithet “bumba” is in reference to “bumba meu boi” or “boi-bumbá”, a folklore character in Northern Brazil. A noun in apposition.
Conservation status. Eigenmannia bumba apparently does not match any of the extinction risk categories giving by International Union for Conservation of Nature (IUCN). Therefore, according with the currently available data, and using the criteria of the IUCN Standards and Petitions Subcommittee (IUCN, 2019), we propose that the species should be classified as Least Concern (LC). Despite being described from only three localities, E. bumba likely possesses a wider distribution in the Mearim basin than reported here.
Eigenmannia cacuria, new species
urn:lsid:zoobank.org:act:CE0B94F3-2259-45F0-88C9-99BA81B3992C
(Figs. 3C, D, 5; Tab. 1)
Eigenmannia virescens (non Valenciennes, 1836). —Ramos et al., 2014:4 (listed, ichthyofauna of the Parnaíba River basin).
Holotype. MZUSP 125871, 110.0 mm LEA, riacho Enxada, Alto Parnaíba, Maranhão, 08°51’21”S 45°59’14”W, O. Oyakawa, A. Akama, V. Garutti & J. Nolasco, 6 Apr 2001.
Paratypes. MZUSP 98583, 4+1CS, 53.6–134.0 mm LEA, collected with holotype. UFPB 11916, 3+1CS, 68.4–90.2 mm LEA, Brejo do Boi, Sambaíba, Maranhão, 06°57’54.3”S 45°22’38.2”W, W. Severi & E. França, 6 Apr 2005.
Non-types. LBP 5572, 10, not measured, stream tributary of rio Parnaíba, Santa Filomena, Piauí, 09°09’51”S 45°51’15”W, C. Oliveira & R. Benine, 28 Nov 2007. UFPB 11896, 2, 55.5–117.5 mm LEA, Brejo do Boi, stream tributary of rio Bois, Sambaíba, Maranhão, 06°57’54”S 45°22’38”W, W. Sereri & E. França, 6 Apr 2005. UFPB 11897, 5, 40.9–63.8 mm LEA, rio Parnaíba, under the bridge of BR-321, Ribeiro Gonçalves, Piauí, 07°33’07.6”S 45°14’13.3”W, W. Sereri & E. França, 31 Mar 2005. UFPB 11898, 2, 53.2–70.4 mm LEA, Riacho Volta, PI-247/BR-324, Uruçuí, Piauí, 07°24’16”S 44°50’31”W, W. Sereri & E. França, 31 Mar 2005. UFPB 11906, 1, 73.5 mm LEA, stream tributary of rio Aldeia, MA-006/BR-330, Balsas, Maranhão, 07°43’50”S 46°02’26”W, W. Sereri & E. França, 3 Apr 2005.
Diagnosis. Eigenmannia cacuria, a member of the E. trilineata species-group, differs from the E. humboldtii species-group by the anal-fin hyaline (vs. anal-fin margin distinctly darkened), and from E. macrops by the absence of enlarged eye (17.5–21.1% HL vs. 26.4–29.7% HL), and the presence of a short caudal filament (26.4–41.3% LEA vs. 67.5–79.3% LEA). Within the E. trilineata species-group,the new species is diagnosed from all species of the E. trilineata species-group, except E. antonioi Peixoto, Dutra & Wosiacki, 2015, E. camposi Herrera-Collazos, Galindo-Cuervo, Maldonado-Ocampo & Ríncon-Sandoval, 2020, E. desantanai Peixoto, Dutra & Wosiacki, 2015, E. guairaca Peixoto, Dutra & Wosiacki, 2015, E. lorenata Waltz & Albert, 2018, E. magoi Herrera-Collazos, Galindo-Cuervo, Maldonado-Ocampo & Ríncon-Sandoval, 2020, E. matintapereira Peixoto, Dutra & Wosiacki, 2015, E. microstoma (Reinhardt, 1852), E. muirapinima Peixoto, Dutra & Wosiacki, 2015, E. pavulagem Peixoto, Dutra & Wosiacki, 2015, E. sayona Peixoto & Waltz, 2017, E. trilineata López & Castello, 1966, and E. zenuensis Herrera-Collazos, Galindo-Cuervo, Maldonado-Ocampo & Ríncon-Sandoval, 2020 by having a terminal mouth (vs. subterminal). Eigenmannia cacuria differs from the aforementioned species, by the following combination of characters: (1) lateral line stripe restricted to last two-thirds of body (vs. lateral line stripe extending from first perforated lateral line scale to distal portion of caudal filament in E. camposi, E. muirapinima, E. zenuensis); (2) ii,11–13 pectoral-fin rays (vs. ii,16–17 in E. matintapereira,and ii,14–15 in E. trilineata); (3) 180–196 anal-fin rays (vs. 216–222 in E. matintapereira,and 198–217 in E. sayona); (4) 12–15 scales rows above lateral line (vs. 8–10 in E. antonioi and E. desantanai, 9–11 in E. guairaca and E. sayona,7–10 in E. camposi, and 7–9 in E. zenuensis); (5) 10–14 premaxillary teeth (vs. 27 in E. camposi, 24–25 in E. desantanai, 32 in E. magoi, 22–24 in E. matintapereira, 16 in E. microstoma, 17 in E. sayona,31–33 in E. trilineata, and 31–34 in E. zenuensis); (6) 15–21 dentary teeth (vs. 35–39 in E. magoi, 25–27 in E. matintapereira, and 56–60 in E. zenuensis); (7) 8–10 endopterygoid teeth (vs. 14–15 in E. desantanai,5–6 in E. guairaca, 6–7 in E. loretana,11 in E. magoi,11–16 in E. microstoma,and 16–17 in E. trilineata); (8) dentary teeth increasing in size along dentigerous surface (vs. all dentary teeth similar in size in E. desantanai, E. guairaca, E. matintapereira, E. microstoma, E. pavulagem, and E. trilineata); (9) basibranchial 1 unossified (vs. ossified in E. sayona); and (10) 14 precaudal vertebrae (vs. 11–12 in E. desantanai,and 15 in E. guairaca).
Description. Body shape and pigmentation shown in Fig. 5, morphometric data in Tab. 1. Largest examined specimen 134.0 mm LEA. Body elongate and distinctly compressed. Greatest body depth at vertical crossing distal tip of pectoral fin. Dorsal profile of body slightly convex from snout tip to vertical through anal-fin terminus. Ventral profile of body convex from tip of lower jaw to vertical through tip of pectoral fin and concave from this point to anal-fin terminus. Caudal filament short.
FIGURE 5| Eigenmannia cacuria, MZUSP 125871, holotype, 110.0 mm LEA, riacho Enxada, Alto Parnaíba, Maranhão, 08°51’21”S 45°59’14”W. A. lateral view of head, B. lateral view of body.
Head laterally compressed; greatest width at opercular region, greatest depth at nape. Dorsal profile of head convex from snout tip to nape. Ventral profile of head convex from tip of lower jaw to isthmus. Snout round in lateral view. Mouth terminal. Mouth rictus at vertical through anterior nostril or between nares. Anterior nostril tube-like; closer to snout tip than to anterior margin of eye. Posterior nostril round, not tubular, closer to anterior margin of eye than to snout tip, at horizontal line above dorsal margin of eye. Eye small, circular, completely covered by skin, on anterior one-half of HL, laterally oriented. Anus adjacent to urogenital papilla, shifting ontogenetically from vertical through posterior margin of opercle to vertical through posterior margin of eye. Urogenital papilla not developed in specimens under 76 mm LEA. Branchial membranes joined at isthmus. Gill rakers on first branchial arch 13(1).
Scales cycloid and small, extending from posterior most part of head to vertical through tip of caudal filament, present on mid-dorsal region of body. Scales above lateral line at vertical through end of pectoral fin 12(1), 13(4), 14(3), or 15*(1). Anterior most perforated lateral-line scale along vertical through pectoral-fin origin. Lateral-line scales to vertical through base of last anal-fin ray 112–132(N = 9), 127 in holotype.
Pectoral-fin rays ii,11(3), ii,12*(6), or ii,13(1). Distal pectoral-fin margin rounded. Total anal-fin rays 180–196(N = 9), 182 in holotype. Anal-fin origin along vertical through pectoral-fin insertion or slightly posterior. Distal margin of anal fin slightly convex. First unbranched rays tiny, subsequent rays progressively increasing in size toward first branched rays. Branched rays of nearly equal length except for posterior most rays that progressively decrease in length.
Relevant osteological features. Premaxillary teeth 10(1) or 14(1) in two(2) rows (Fig. 3C). Dentary teeth 15(1), or 21(1) in two(2) rows (Fig. 3D). Dentary teeth increasing in size along dentigerous surface. Coronomeckelian bone length near 20% Meckel’s cartilage length. Endopterygoid teeth eight(1), or 10(1) in one(1) or two(1) rows. Antorbital and infraorbitals 1 to 4 enlarged, partially cylindrical with slender osseous arches. Fifth and sixth infraorbitals slender and tubular. Depth of posterodorsal expansion on infraorbitals 1+2 half as long as infraorbitals 1+2 length. Branchiostegals four(1) or five(1). Upper pharyngeal teeth nine(1). Lower pharyngeal teeth nine(1). Precaudal vertebrae 14(1). Transitional vertebrae five(1). Pleural ribs seven(1). Displaced hemal spines three(1).
Coloration in alcohol. Body ground coloration cream. Body with two layers of chromatophores. Outer layer covered by dark chromatophores gradually more spaced ventrally, more concentrated on perforated scales forming a lateral line stripe, and between lateral line stripe and anal pterygiophores forming a superior midlateral stripe. Lateral line stripe restricted to last two thirds of body. Superior medial stripe fainted, covering the space equivalent to two scales vertically, tapering from vertical through end of body cavity to vertical through to posterior one-third of LEA. Inner layer of pigmentation formed by multiple, small bars of dark chromatophores situated between the musculature associated with anal-fin pterygiophores. Dark individual bars in combination form two stripes-like patterns. Inferior midlateral stripe fainted approximately half as wide as orbital diameter. Anal-fin base stripe approximately half as wide as orbital diameter. Head covered by dark chromatophores. Pectoral and anal fins hyaline with scattered dark chromatophores overlying fin rays.
Geographical distribution. Eigenmannia cacuria is only known from the upper Rio Parnaíba basin, Northeastern Brazil. It occurs in the main course of Rio Parnaíba, and in small streams tributaries of Rio Balsas, the largest tributary of the basin (Fig. 4).
Ecological notes. Eigenmannia cacuria is apparently restricted to perennial rivers. Specimens were collected in a lentic environment, with clear water, sandy substrate, and varying amounts of remnants of riparian vegetation typical of the Cerrado biome, with palms of the species Mauritia flexuosa.
Etymology. The epithet “cacuria” is in reference to the “cacuriá”, a typical dance in the state of Maranhão, where the holotype was collected. A noun in apposition.
Conservation status. Eigenmannia cacuria apparently does not match any of the extinction risk categories giving by the International Union for Conservation of Nature (IUCN). The species possesses a relatively broad distribution, occurring at the upper portion of the Rio Parnaíba basin. Therefore, according with the currently available data, and using the criteria of the IUCN Standards and Petitions Subcommittee (IUCN, 2019), we propose that the species should be classified as Least Concern (LC). However, we draw attention to the low number of known specimens of this species in a relatively well sampled region.
Eigenmannia robsoni, new species
urn:lsid:zoobank.org:act:A0CD04E8-6A7A-49B0-8D9A-580B8A7B85D0
(Figs. 3E, F, 6; Tab. 1)
Eigenmannia virescens (non Valenciennes, 1836). —Fowler, 1941:196 (listed, Rio Parnahyba, Therezina, Piauhy). —Silva et al., 2015:5 (listed, ichthyofauna of the Gurgueia River). —Melo et al., 2016:374 (listed, ichthyofauna of lower Parnaíba River).
Eigenmannia macrops (non Boulenger, 1897). —Ramos et al., 2014:4 (listed, ichthyofauna of the Parnaíba River basin).
Holotype. MZUSP 125872, 139.3 mm LEA, rio Parnaíba, Muricí dos Portelas, Piauí, 03°18’24.8”S 42°05’36.2”W, T. Ramos & S. Ramos, 10 Apr 2010.
Paratypes. All from Brazil, rio Parnaíba basin: UFPB 8204, 4+1CS, 121.3–130.8 mm LEA, collected with holotype. MZUSP 74875, 2, 83.8–100.2 mm LEA, rio Poti near the Parque Municipal da Floresta Fóssil, Teresina, Piauí, 05°05’14.5”S 42°47’19.4”W, M. de Pinna, 16 Nov 2001. MZUSP 87481, 1, 201.8 mm LEA, ribeirão Jenipapo, Balsas, Maranhão, 07°26’18”S 46°11’47”W, A. Akama & E. Baena, 23 Mar 2005. UFPB 8201, 5, 86.6–109.5 mm LEA, rio Parnaíba at Community of Beira Rio, Buriti, Maranhão, 03°54’06.9”S 42°43’27.8”W, T. Ramos & S. Ramos, 9 Apr 2010. UFPB 8202, 7+1CS, 88.4–134.5 mm LEA, rio Parnaíba, Magalhães de Almeida, Maranhão, 03°18’24.8”S 42°05’36.0”W, T. Ramos & S. Ramos, 10 Apr 2010. UFPB 10014, 1, 122.8 mm LEA, rio Gurgueia, Cristiano Castro, Piauí, 08°48’55.7”S 44°13’47.9”W, W. Severi et al., 22 Jun 2006.
Non-types. MZUSP 5110, 1, 203.9 mm LEA, Teresina Market, Expedição do Departamento de Zoologia, 19 Jun 1966. MZUSP 97958, 1, not measured, Isle at Lagoa de Parnaguá, Parnaguá, Piauí, 10°14’41”S 44°38’50”W, O. Oyakawa, A. Akama, V. Garutti & J. Nolasco, 8 Apr 2001. MZUSP 98596, 1, 21.2 mm LEA, Rio Balsas, Balsas, Maranhão, 07°32’13”S 46°02’20”W, O. Oyakawa, A. Akama, V. Garutti & J. Nolasco, 6 Apr 2001. UFPB 8196, 4, 82.2–128.3 mm LEA, Rio Parnaíba, Ribeiro Gonçalves, Piauí, 07°33’24”S 45°14’58”W, T. Ramos & S. Ramos, 3 Apr 2010. UFPB 8197, 1, 54.0 mm LEA, rio Parnaíba, Ribeiro Gonçalves, Maranhão, 07°33’07”S 45°14’18” W, T. Ramos & S. Ramos, 3 Apr 2010. UFPB 8198, 2, 55.15–92.3 mm LEA, rio Parnaíba, Benedito Leite, Piauí, 07°14’43”S 44°34’16”W, T. Ramos & S. Ramos, 4 Apr 2010. UFPB 8199, 4, 76.8–198.6 mm LEA, rio Parnaíba, Uruçuí, Piauí, 07°14’22”S 44°34’08”W, T. Ramos & S. Ramos, 4 Apr 2010. UFPB 8200, 4, 59.0–126.2 mm LEA, Rio Parnaíba, village of Correntes, Caxias, Maranhão, 04°33’42”S 42°52’01”W, T. Ramos & S. Ramos, 8 Apr 2010. UFPB 11899, 1, 57.8 mm LEA, Riacho Nova Brasília, stream tributary of Santo Antônio, village of Nova Brasília, rod. PI-113, Cabeceiras do Piauí, Piauí, 04°27’14”S 42°18’47”W, W. Severi & E. França, 18 Jun 2006. UFPB 11900, 4, 166.1–225.1 mm LEA, Mercado Público, União, Piauí, 04°35’24”S 42°52’31”W, T. Ramos, 24 Sep 2009. UFPB 11901, 21, 75.2–154.3 mm LEA, rio Gurguéia, Jurumenha, Piauí, 07º04’36”S 43º30’54”W, W. Severi & E. França, 7 Apr 2005. UFPB 11902, 2, 113.4–134.5 mm LEA, Barragem Boa Esperança, São João dos Patos, Maranhão, 06°39’29”S 43°42’34”W, T. Ramos, R. Ramos & G. Moro, 3 Feb 2009. UFPB 11903, 2, 143.3–203.4 mm LEA, rio Parnaíba, Benedito Leite, Maranhão, 07°14’09”S 44°34’24”W, W. Severi & E. França, 1 Apr 2005. UFPB 11904, 7, 77.5–134.3 mm LEA, rio Parnaíba, Alto Parnaíba, Maranhão, 09°06’52”S 45°55’35”W, T. Ramos & S. Ramos, 1 Apr 2010. UFPB 11905, 1, 92.3 mm LEA, rio Canindé, Francisco Aires, Piauí, 06°37’28.7”S 42°41’43”W, T. Ramos, G. Moro & M. Silva, 19 Sep 2009. UFPB 11907, 1, 64.4 mm LEA, rio Parnaíba, Uruçuí, Piauí, 07°14’11”S 44°34’03”W, W. Severi & E. França, 1 Apr 2005. UFPB 11908, 2, 72.8–108.4 mm LEA, rio Uruçui-Preto, PI-247/BR-324, Uruçuí, Piauí, 07°23’19”S 44°36’42”W, W. Severi & E. França, 31 Mar 2005. UFPB 11909, 1, 138.3 mm LEA, rio Parnaíba, Alto Parnaíba, Maranhão, 09°06’53”S 45°55’37”W, T. Ramos & S. Ramos, 6 Feb 2009. UFPB 11910, 1, 122.1 mm LEA, rio Parnaíba, village of Beira-Rio, Buriti, Maranhão, 03°53’39”S 42°43’25”W, T. Ramos & S. Ramos, 17 Apr 2011. UFPB 11911, 1, 121.1 mm LEA, rio Parnaíba, village of Beira-rio, Buriti, Maranhão, 03°53’39”S 42°43’25”W, T. Ramos & S. Ramos, 17 Apr 2011. UFPB 11912, 5, 82.5–153.4 mm LEA, rio Parnaíba, União, Piauí, 04°34’27”S 42°52’13”W, T. Ramos & S. Ramos, 18 Apr 2011. UFPB 11913, 3, 71.2–97.7 mm LEA, rio Parnaíba, village of Manga, Barão do Grajaú, Maranhão, 06°14’47”S 42°51’24”W, T. Ramos & S. Ramos, 18 Apr 2011. UFPB 11914, 1, 115.9 mm LEA, rio Parnaíba, Murici dos Portelas, Piauí, 03°18’24”S 42°05’36”W, T. Ramos & S. Ramos, 16 Apr 2011. UFPB 11915, 37, 61.2–97.9 mm LEA, rio Longá, Nossa Senhora de Nazaré, Piauí, 04°40’20”S 42°13’W, T. Ramos & S. Ramos, 23 Jun 2011. UFPB 11921, 2, 86.1–95.1 mm LEA, rio Titara, Cocal de Telha, Piauí, 04°39’39”S 42°03’41.7”W, T. Ramos, S. Costa, Y. Rocha & L. Neto, 8 Dec 2018.
Diagnosis. Eigenmannia robsoni, a member of the E. trilineata species-group, differs from the E. humboldtii species-group by the anal-fin hyaline (vs. anal-fin margin distinctly darkened), and from E. macrops by the absence of enlarged eye (16.2–21.3% HL vs. 26.4–29.7% HL), and the presence of a short caudal filament (19.9–36.4 % LEA vs. 67.5–79.3% LEA). The new species differs from all other species of the E. trilineata species-group, except E. besouro, E. bumba, E. correntes, E. dutrai, E. guchereauae, E. meeki, E. oradens, E. sirius, E. vicentespeleaea, E. virescens, and E. waiwai by the subterminal mouth (vs. terminal). Eigenmannia robsoni differs from aforementioned species by the following combination of characters: (1) absence of the lateral line stripe (vs. presence in E. besouro, E. correntes, E. dutrai, E. guchereauae, E. meeki, E. oradens, E. sirius, E. vicentespelaea, and E. waiwai); (2) absence of superior midlateral stripe (vs. presence in E. besouro, E. bumba, E. correntes, E. dutrai, E. sirius, and E. vicentespeleaea); (3) 182–228 anal-fin rays (vs. 143–154 in E. correntes); (4) 12–15 scales rows above lateral line (vs. 7–10 in E. besouro, 8–11 in E. oradens,7–8in E. vicentespelaea, 9–11 in E. virescens, and 9–10 in E. waiwai); (5) 107–131 scales on lateral line (vs. 140–168 in E. meeki), (6) 32–34 premaxillary teeth (vs. 19–23 in E. bumba, 11–20 in E. correntes, 75 in E. guchereauae, 38–42 in E. oradens, 15–24 in E. sirius,25–26 in E. vicentespeleaea, and 22 in E. virescens); (7) 35–44 dentary teeth (vs. 19–30 in E. besouro,20–29 in E. bumba, 16–18 in E. correntes, 88 in E. guchereauae, and 21–23 in E. meeki); (8) 9–12 endopterygoid teeth (vs. 13–15 in E. meeki,14–17 in E. waiwai); (9) depth of posterodorsal expansion on infraorbitals 1+2 half as long as infraorbitals 1+2 length (vs. as long as infraorbitals 1+2 length in E. dutrai, E. guchereauae, E. oradens, E. sirius); (10) basibranchial 1 unossified (vs. ossified in E. virescens); (11) 13 precaudal vertebrae (vs. 15 in E. meeki and E. sirius); (12) coronomeckelian bone length corresponding to 20% of length of Meckel’s cartilage (vs. 45% in E. oradens and E. waiwai).
Description. Body shape and pigmentation shown in Fig. 6, morphometric data in Tab. 1. Largest examined specimen 225.1 mm LEA. Body elongate and distinctly compressed laterally. Greatest body depth at vertical crossing distal tip of pectoral fin. Dorsal profile of body slightly convex from snout tip to vertical through anal-fin terminus. Ventral profile of body nearly straight from tip of lower jaw to vertical through tip of pectoral fin and concave from this point to anal-fin terminus. Caudal filament short (19.9–36.4% LEA).
FIGURE 6| Eigenmannia robsoni, MZUSP 125872, holotype, 139.3 mm LEA, rio Parnaíba, Muricí dos Portelas, Piauí, 03°18’24.8”S 42°05’36.2”W. A. lateral view of head, B. lateral view of body.
Head laterally compressed; greatest width at opercular region, greatest depth at nape. Dorsal profile of head convex from snout tip to nape. Ventral profile of head nearly straight from tip of lower jaw to isthmus. Snout pointed in lateral view. Mouth subterminal. Mouth rictus at vertical through a point between anterior and posterior nares or vertical through posterior nostril. Anterior nostril tube-like; closer to snout tip than to anterior margin of eye. Posterior nostril round, not tubular, closer to anterior margin of eye than to snout tip, at horizontal line between middle and dorsal margin of eye. Eye small, circular, completely covered by skin, on anterior one-half of HL, laterally oriented. Anus adjacent to urogenital papilla, shifting ontogenetically from vertical through middle of opercle to vertical through posterior margin of eye. Urogenital papilla usually not developed in specimens under 111 mm LEA. Branchial membranes joined at isthmus. Gill rakers on first branchial arch 12(1).
Scales cycloid and small, extending from posterior most part of head to vertical through tip of caudal filament, present on mid-dorsal region of body. Scales above lateral line at vertical through end of pectoral fin 12(1), 13*(8), 14(5), or 15(4). Anterior most perforated lateral-line scale along vertical through pectoral-fin origin. Lateral-line scales to vertical through base of last anal-fin ray 107–131(N = 17), 126 in holotype.
Pectoral-fin rays ii,12(2), ii,13(1), ii,14(14), or ii,15*(2). Distal pectoral-fin margin straight. Total anal-fin rays 182–228(N = 17), 216 in holotype. Anal-fin origin along vertical through pectoral-fin insertion or slightly posterior. Distal margin of anal fin slightly convex. First unbranched rays tiny, subsequent rays progressively increasing in size toward first branched rays. Branched rays of nearly equal length except for posterior most rays that progressively decrease in length.
Relevant osteological features. Premaxillary teeth 32(1) or 34(1) in five rows (Fig. 3E). Dentary teeth 35(1), or 44(1) in three(2) rows (Fig. 3F). Dentary teeth not increasing in size along dentigerous surface. Coronomeckelian bone length near 20% Meckel’s cartilage length. Endopterygoid teeth nine(1) or 12(1) in one(1) or three(1) rows. Antorbital and infraorbitals 1 to 4 enlarged, partially cylindrical with slender osseous arches. Fifth and sixth infraorbitals slender and tubular. Depth of posterodorsal expansion on infraorbitals 1+2 half as long as infraorbitals 1+2 length. Branchiostegals five(2). Upper pharyngeal teeth eight(1). Lower pharyngeal teeth 12(1). Precaudal vertebrae 13(1). Transitional vertebrae three(1). Pleural ribs seven(1). Displaced hemal spines three(1).
Coloration in alcohol. Body ground coloration cream. Body with two layers of chromatophores, outer layer covered by dark chromatophores gradually more spaced ventrally. Inner layer of pigmentation formed by multiple, small bars of dark chromatophores situated between the musculature associated with anal-fin pterygiophores. Dark individual bars in combination forming stripe-like pattern on anal-fin base. Anal-fin base stripe approximately half as wide as orbital diameter. Head covered by dark chromatophores, more concentrated on opercular region. Pectoral and anal fins hyaline with scattered dark chromatophores overlying fin rays.
Geographical distribution. Eigenmannia robsoni is widespread in the rio Parnaíba basin, Northeastern Brazil. This species occurs in the main course of rio Parnaíba, and in its tributaries rio Balsas, rio Gurgueia, rio Longá, rio Piauí-Canindé, and rio Poti (Fig. 4).
Ecological notes. Eigenmannia robsoni is recorded in perennial and intermittent rivers with clear and lentic waters, sandy substrate with lots of aquatic vegetation. In perennial rivers, the riparian vegetation is typical of the Cerrado, with palms of the species Mauritia flexuosa. In contrast, in intermittent rivers, the riparian vegetation is the “fringe forests”, dominated by palm trees such as Copernicia prunifera.
Etymology. The epithet “robsoni” is in honor of Robson Tamar da Costa Ramos, ichthyologist, for his contributions to studies of the Caatinga ecoregion, especially in the Parnaíba river basin. A patronym.
Conservation status. Eigenmannia robsoni apparently does not match any of the extinction risk categories giving by the International Union for Conservation of Nature (IUCN). The species possesses a relatively broad distribution, being widespread in the Rio Parnaíba basin. Therefore, according with the currently available data, and using the criteria of the IUCN Standards and Petitions Subcommittee (IUCN, 2019), we propose that the species should be classified as Least Concern (LC).
Remarks. Eigenmannia robsoni was initially identified as E. macrops by Ramos et al. (2014). Such identification was based on the lack of lateral stripes, which are also absent in E. macrops. However, E. robsoni can be readily diagnosed from E. macrops as described in the diagnosis.
Key to the species of Eigenmannia from rio Mearim and rio Parnaíba basins
1a. Mouth subterminal, snout pointed in lateral view, posterior nostril at horizontal line between middle and dorsal margin of eye, pectoral-fin margin straight, ii,12–15 pectoral-fin rays…………………2
1b. Mouth terminal, snout rounded in lateral view, posterior nostril at horizontal line above dorsal margin of eye, pectoral-fin margin rounded, ii,11–13 pectoral-fin rays…………………Eigenmannia cacuria (rio Parnaíba basin)
2a. Superior midlateral stripe present, 19–23 premaxillary teeth, 20–29 dentary teeth…………………Eigenmannia bumba (rio Mearim basin)
2b. Superior midlateral stripe absent, 32–34 premaxillary teeth, 35–44 dentary teeth…………………Eigenmannia robsoni (rio Parnaíba basin)
Discussion
The Eigenmannia trilineata species-group was initially proposed by Peixoto et al. (2015) to include species with a superior midlateral stripe. More recently, however, as a result of a phylogenetic analysis, this group was redefined to comprises species that possess the lateral-line stripe (Dutra et al., 2021). Further, that analysis revealed that both of these stripes may be absent in some members of the Eigenmannia trilineata species-group, as in E. virescens, which lack both stripes, or in E. guchereauae and E. oradens, in which the superior midlateral stripe is absent. In these cases, the absence of stripes was interpreted as a secondary loss, considering that they are nested within the E. trilineata species-group clade (see Dutra et al., 2021: fig. 47). Neverthless, members of E. trilineata species-group can be also diagnosed from E. humboltdi species-group (which includes E. humboldti (Steindachner, 1878), E. limbata (Schreiner & Miranda Ribeiro, 1903), and E. nigra Mago-Leccia, 1994 by the absence of anal-fin margin distinctly darkened, and from the E. macrops (which do not belong to any group) by the absence of enlarged eyes and the short caudal filament (Waltz, Albert, 2017; Peixoto, Ohara, 2019; Dutra et al., 2021).
Among the newly discovered species, the lateral-line and the superior midlateral stripes are present in E. bumba and E. cacuria. In the other hand, these stripes are absent in E. robsoni. Further, none of these species possess the diagnostic features for the Eigenmannia humboltdi species-group nor for E. macrops.Thus, all described species herein are recognized as a members of E. trilineata species-group, and the absences of lateral-line and the superior midlateral stripes in E. robsoni is interpreted here as a secondary lost.
The Eigenmannia species from the Mearim and Parnaíba river basins had been historically identified as E. virescens by several authors (Fowler, 1941; Piorski, 1998; Ramos et al., 2014; Silva et al., 2015; Melo et al., 2016; Brito et al., 2019; Guimarães et al., 2020). This is not surprising, because until recently, E. virescens was considered to be a widespread species in South America (e.g., Ellis, 1913; Meek, Hildebrand, 1916; Fowler, 1941; Mago-Leccia, 1978, 1994; Albert, 2003). Campos-da-Paz (2007) instead argued that E. virescens occurs only in the Paraná-Paraguay basin, and this hypothesis was corroborated and posteriorly refined by Peixoto et al. (2015) who postulate that the Río de La Plata is the probable type locality of E. virescens, and that its range is restricted to the lower Paraná and La Plata basins.
The description of E. bumba, E. cacuria and E. robsoni, adds three additional endemic species for northeastern Brazil and highlight advances in the ongoing taxonomic revision of the diverse genus Eigenmannia.
Comparative material. In addition to the comparative material examined listed in Dutra et al. (2014, 2017, 2018, 2021), the following species were examined: Eigenmannia camposi: All from Colombia, Río Magdalena. IAvH-P 7819, 6 of 19 paratypes, 119.0–134.7 mm LEA. IAvH-P 7820, 1 of 6 paratypes, 153.6 mm LEA. IAvH-P 7023, 1, 107,3 mm LEA. USNM 330526, 2, 96.1–115.7 mm LEA. Eigenmannia dutrai: All from Brazil, upper rio Paraná basin: MZUSP 125788, holotype, 203.8 mm LEA, rio Passa Cinco, LBP 471, 1 paratype, 172.3 mm LEA, rio Passa Cinco. MZUSP 87940, 4+2CS, 112.7–130 mm LEA, rio Tiête. MZUSP 49383, 2 paratypes, 102.0–162.9 mm LEA, Córrego das Araras. MZUSP 118377, 1 paratype, 145.6 mm LEA, Córrego do Canta Galo. Eigenmannia macrops: All from Guyana: BMNH 1897.8.6.1, holotype, 128.5 mm LEA, Higher Potaro River District. ROM 79090, 1, 134.0 mm LEA, Mazaruni River. ROM 79092, 1, 104.6 mm LEA, Mazaruni River. Eigenmannia magoi: LBP 6112, 12, 91.1–130.7 mm LEA, Río Santa Rosa, Lago Maracaibo basin, Venezuela. Eigenmannia sirius: All from Brazil, rio Tapajós basin, rio Mutum: MZUSP 121668, holotype, 127.5 mm LEA. MPEG 34989, 1 paratype, 92.3 mm LEA. MPEG 34990, 1 paratype, 88.3 mm LEA. MZUSP 118579, 2 paratypes, 102.7–106.2 mm LEA. MZUSP 118580, 2 paratypes, 87.6–115.9 mm LEA, MZUSP 118581, 6+2CS paratypes, 58.8–110.5 mm LEA. MZUSP 118582, 10+2CS paratypes, 86.6–93.3 mm LEA. MZUSP 123938, 7 paratypes, 51.0–93.7 mm LEA.
Acknowledgments
Authors are thankful to the following individuals and institutions for access to material under their care: B. Brown (AMNH), M. Sabaj and M. Arce (ANSP), J. Maclaine (BMNH), D. Catania (CAS), C. McMahan and S. Mochel (FMNH), C. Uribe (IAvH), L. Rapp Py-Daniel and R. de Oliveira (INPA), C. Oliveira (LBP), G. Santos and T. Pessali (MCNIP), C. Lucena and Z. Lucena (MCP); K. Hartel (MCZ), M. Britto and P. Buckup (MNRJ), W. Wosiacki and I. Maschio (MPEG), C. Pavanelli (NUP), M. Marinho (UFPB), L. Malabarba (UFRGS), and L. Parenti, S. Raredon, K. Murphy and J. Clayton (USNM). G. Vita (MZUSP) assisted us with the photographs of the holotypes. M. Mendonça (UFPA) kindly provided information on the type of Eigenmannia macrops. GMD was financially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (grant #2018/09445–9). This contribution was also supported by the Diversity and Evolution of Gymnotiformes Project (FAPESP/Smithsonian proc. 2016/19075–9), and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
References
Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N, Coad B, Mandrak N, Balderas SC, Bussing W, Stiassny MLJ, Skelton P, Allen GR, Unmack P, Naseka A, Ng R, Sindorf N, Robertson J, Armijo E, Higgins JV, Heibelv TJ, Wikramanayake E, Olson D, López HL, Reis RE, Lundberg JG, Sabaj Pérez MH, Petry P. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. Bioscience. 2008; 58(5):403–14. https://doi.org/10.1641/B580507
Albert JS. Family Sternopygidae. In: Reis RE, Kullander SO, Ferraris, Jr. CJ, editors. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs; 2003.
Alda F, Tagliacollo VA, Bernt MJ, Waltz BT, Ludt WB, Faircloth BC, Alfaro ME, Albert JS, Chakrabarty P. Resolving deep nodes in an ancient radiation of Neotropical fishes in the presence of conflicting signals from incomplete lineage sorting. Syst Biol. 2019; 68(4):573–93. http://doi.org/10.1093/sysbio/syy085
Brito PS, Guimarães EC, Ferreira BRA, Ottoni FP, Piorski NM. Freshwater fishes of the Parque Nacional dos Lençóis Maranhenses and adjacent areas. Biota Neotrop. 2019; 19(3):e20180660. http://dx.doi.org/10.1590/1676-0611-BN-2018-0660.
Campos-da-Paz R. Family Sternopygidae. In: Buckup PA, Menezes NA, Ghazzi MS, editors. Catálogo das espécies de peixes de água doce do Brasil. Rio de Janeiro: Museu Nacional; 2007. p.121–22.
Campos-da-Paz R, Queiroz IR. A new species of Eigenmannia Jordan and Evermann (Gymnotiformes: Sternopygidae) from the upper rio Paraguai basin. Zootaxa. 2017; 4216(1):73–84. http://doi.org/10.11646/zootaxa.4216.1.5
Dutra GM, Peixoto LAW, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD. Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. J Zool Syst Evol Res. 2021; 59(8):2010–59. http://doi.org/10.1111/jzs.12535
Dutra GM, Peixoto LAW, de Santana CD, Wosiacki WB. A new species of Eigenmannia Jordan, Evermann (Teleostei: Gymnotiformes: Sternopygidae) from Río Ventuari, Venezuela. Zootaxa. 2018; 4422(1):132–40. https://doi.org/10.11646/zootaxa.4422.1.8
Dutra GM, de Santana CD, Vari RP, Wosiacki WB. The South American electric glass knifefish genus Distocyclus (Gymnotiformes: Sternopygidae): Redefinition and revision. Copeia. 2014; 2014(2):345–54. http://doi.org/10.1643/CI-13-066
Dutra GM, de Santana CD, Wosiacki WB. A new species of the glass electric knifefish genus Eigenmannia Jordan and Evermann (Teleostei: Gymnotiformes: Sternopygidae) from Río Tuíra basin, Panama. Copeia. 2017; 105(1):85–91. https://doi.org/10.1643/CI-16-439
Ellis MM. The gymnotid eels of tropical America. Mem Carnegie Mus. 1913; 6(3):109–95. https://doi.org/10.5962/bhl.title.12969
Ferraris CJ, Jr., de Santana CD, Vari RP. Checklist of Gymnotiformes (Osteichthyes: Ostariophysi) and catalogue of primary types. Neotrop Ichthyol. 2017; 15(1):e160067. https://doi.org/10.1590/1982-0224-20160067
Fowler HW. A collection of fresh-water fishes obtained in eastern Brazil by Dr. Rodolpho Von Ihering. Proc Acad Nat Sci Phila. 1941; 93:123–336. https://www.jstor.org/stable/4064332
Guimarães EC, Brito PS, Gonçalves CS, Ottoni FP. An inventory of ichthyofauna of the Pindaré river drainage, Mearim river basin, northeastern Brazil. Biota Neotrop. 2020; 20(4):e20201023. https://doi.org/10.1590/1676-0611-BN-2020-1023
Herrera-Collazos EE, Galindo-Cuervo AM, Maldonado-Ocampo JA, Rincón-Sandoval M. Three new species of the Eigenmannia trilineata species group (Gymnotiformes: Sternopygidae) from northwestern South America. Neotrop Ichthyol. 2020; 18(1):e180085. https://doi.org/10.1590/10.1590/1982-0224-2018-0085
Hopkins CD. Hypopomus pinnicaudatus (Hypopomidae), a new species of gymnotiform fish from French Guiana. Copeia. 1991; 1991(1):151–61. https://doi.org/10.2307/1446259
International Union for Conservation of Nature (IUCN). Standards and petitions subcommittee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Standards and Petitions Subcommittee; 2019. http://www.iucnredlist.org/documents/RedListGuidelines.pdf
Mago-Leccia F. Los peces de la familia Sternopygidae de Venezuela. Acta Cient Venez. 1978; 29(1):1–89.
Mago-Leccia F. Electric fishes of the continental waters of America. Caracas, Fundacion para el Desarrollo de las Ciencias Fisicas, Matematicas y Naturales. Caracas, FUDECI. 1994.
Meek SE, Hildebrand SF. The fishes of the freshwaters of Panama. Field Mus Nat Hist Zool. 1916; 10(15):217–374. https://doi.org/10.5962/bhl.title.2576
Melo FAG, Buckup PA, Ramos TPA, Souza AKN, Silva CMA, Costa TC, Torres AR. Fish fauna of the lower course of the Parnaíba river, northeastern Brazil. Bol Mus Biol Mello Leitão, Nova Sér. 2016; 38(4):363–400. http://boletim.sambio.org.br/pdf/38_4_05.pdf
Peixoto LAW, Dutra GM, Wosiacki WB. The electric glass knifefishes of the Eigenmannia trilineata species-group (Gymnotiformes: Sternopygidae): Monophyly and description of seven new species. Zool J Linn Soc. 2015; 175(2):384–414. https://doi.org/10.1111/zoj.12274
Peixoto LAW, Ohara WM. A new species of Eigenmannia Jordan & Evermann (Gymnotiformes: Sternopygidae) from rio Tapajós, Brazil, with discussion on its species group and the myology within Eigenmanniinae. PLoS ONE. 2019; 14(8):e0220287. https://doi.org/10.1371/journal.pone.0220287
Peixoto LAW, Waltz BT. A new species of the Eigenmannia trilineata species-group from the río Orinoco basin, Venezuela (Gymnotiformes: Sternopygidae). Neotrop Ichthyol. 2017; 15(1):e150199. https://doi.org/10.1590/1982-0224-20150199
Peixoto LAW, Wosiacki WB. Eigenmannia besouro, a new species of the Eigenmannia trilineata species-group (Gymnotiformes: Sternopygidae) from the rio São Francisco basin, northeastern Brazil. Zootaxa. 2016; 4126(2):262–70. https://doi.org/10.11646/zootaxa.4126.2.6
Peixoto LAW, Pastana MN, Ballen GA. New species of glass knifefish genus Eigenmannia (Gymnotiformes: Sternopygidae) with comments on the morphology and function of the enlarged cephalic lateral-line canals of Sternopygidae. J Fish Biol. 2021; 98(1):142–53. https://doi.org/10.1111/jfb.14564
Piorski NM, Castro ACL, Pereira LG, Muniz MEL. Ictiofauna do trecho inferior do Rio Itapecuru, nordeste do Brasil. Bol Lab Hidrobiol. 1998; 11(1):15–24. http://www.periodicoseletronicos.ufma.br/index.php/blabohidro/article/view/2011
Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river basin, northeastern Brazil. Biota Neotrop. 2014; 14(1):e20130039. https://doi.org/10.1590/S1676-06020140039
Sabaj MH. Standard symbolic codes for institutional resource collections in herpetology and ichthyology. Version 7.1 [Internet]. Washington (DC): ASIH; 2019.
Silva MJ, Costa BG, Ramos TPA, Auricchio P, Lima SMQ. Ichthyofauna of the Gurgueia River, Parnaíba River basin, northeastern Brazil. Check List. 2015; 11(5):1765. https://doi.org/10.15560/11.5.1765
Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–19.
Waltz BT, Albert JS. Family Sternopygidae: Glass knifefishes, rattail knifefishes. In: van der Sleen P, Albert JS, editors. Field guide to the fishes of the Amazon, Orinoco & Guianas. Princeton: Princeton University Press; 2017. p.341–45.
Waltz BT, Albert JS. New species of glass knifefish Eigenmannia loretana (Gymnotiformes: Sternopygidae) from the Western Amazon. Zootaxa. 2018; 4399(3):399–411. https://doi.org/10.11646/zootaxa.4399.3.9
Authors
![]() Guilherme Moreira Dutra1
Guilherme Moreira Dutra1 ![]() ,
, ![]() Telton Pedro Anselmo Ramos2 and
Telton Pedro Anselmo Ramos2 and ![]() Naércio Aquino Menezes1
Naércio Aquino Menezes1
[1] Museu de Zoologia da Universidade de São Paulo, Av. Nazaré, 481, Ipiranga, 04263-000 São Paulo, SP, Brazil. (GMD) guilhermedutr@yahoo.com.br (corresponding author), (NAM) naercio@usp.br.
[2] Universidade Estadual da Paraíba, Laboratório de Ecologia Aquática, Departamento de Biologia/CCBS, Rua Baraúnas, 351, Universitário, 58429-500 Campina Grande, PB, Brazil. telton@gmail.com.
Authors’ Contribution 


Guilherme Moreira Dutra: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing-original draft, Writing-review and editing.
Telton Pedro Anselmo Ramos: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing-original draft, Writing-review and editing.
Naércio Aquino Menezes: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing-original draft, Writing-review and editing.
Ethical Statement
Not applicable.
Competing Interests
The authors declare no competing interests.
How to cite this article
Dutra GM, Ramos TPA, Menezes NA. Description of three new species of Eigenmannia (Gymnotiformes: Sternopygidae) from the rio Mearim and rio Parnaíba basins, Northeastern Brazil. Neotrop Ichthyol. 2022; 20(1):e210117. https://doi.org/10.1590/1982-0224-2021-0117
Copyright
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Distributed under
Creative Commons CC-BY 4.0

© 2022 The Authors.
Diversity and Distributions Published by SBI
![]() Accepted January 25, 2022 by William Crampton
Accepted January 25, 2022 by William Crampton
![]() Submitted July 22, 2021
Submitted July 22, 2021
![]() Epub April 1, 2022
Epub April 1, 2022